Abstract
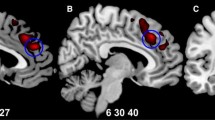
This study aims to examine regional gray matter (GM) changes over a period of 2 years in patients diagnosed with early-onset first-episode psychosis (EO-FEP), and to identify baseline predictors of abnormalities at the follow-up. Fifty-nine patients with EO-FEP aged 11–17 years were assessed. Magnetic resonance imaging was carried out at admission and 2 years later. Changes over time were assessed with voxel-based morphometry. Fifty-nine patients (34 schizophrenia—SCZ, 15 bipolar disorder—BP, and 10 other psychotic disorders) and 70 healthy controls were assessed. At baseline no differences were found between the EO-FEP groups and control subjects. Over time, SCZ patients presented a larger GM decrease in the orbitofrontal cortex, anterior midline frontal cortex, cingulate, left caudate, and thalamus. BP patients also had a larger GM decrease in the right putamen, right orbitofrontal cortex, and anterior and midline region of the right superior frontal gyrus and left caudate, but with fewer areas showing significant differences than in the comparison between SCZ and controls. In the cross-sectional analysis, only SCZ patients showed differences with respect to controls in some GM areas. Significant baseline predictors of a 2-year reduction in GM were IQ and working memory. EO-FEP patients did not show differences in GM compared to controls at baseline. Both SCZ and BP patients showed a greater decrease in specific areas during the first 2 years. At follow-up, only SCZ patients differed significantly from controls in specific brain areas. The GM reduction was predicted by baseline cognitive variables.



Similar content being viewed by others
References
Andreasen NC, Nopoulos P, Magnotta V et al (2011) Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 70:672–679
Haijma SV, Van Haren N, Cahn W et al (2013) Brain volumes in schizophrenia: a meta-analysis in over 18,000 subjects. Schizophr Bull 39:1129–1138
Vidal CN, Rapoport JL, Hayashi KM et al (2006) Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry 63:25–34
Gogtay N (2008) Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull 34:30–36
Pagsberg AK, Baare WF, Raabjerg Christensen AM et al (2007) Structural brain abnormalities in early onset first-episode psychosis. J Neural Transm 114:489–498
Janssen J, Reig S, Parellada M et al (2008) Regional gray matter volume deficits in adolescents with first-episode psychosis. J Am Acad Child Adolesc Psychiatry 47:1311–1320
Tang J, Liao Y, Zhou B et al (2012) Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PLoS ONE 7:e40247
Reig S, Moreno C, Moreno D et al (2009) Progression of brain volume changes in adolescent-onset psychosis. Schizophr Bull 35:233–243
Arango C, Rapado-Castro M, Reig S et al (2012) Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry 69:16–26. doi:10.1001/archgenpsychiatry.2011.150
Fraguas D, Díaz-Caneja CM, Pina-Camacho L et al (2014) Progressive brain changes in children and adolescents with early-onset psychosis: a meta-analysis of longitudinal MRI studies. Schizophr Res 173:132–139
Ayesa-Arriola R, Roiz-Santianez R, Perez-Iglesias R et al (2013) Neuroanatomical differences between first-episode psychosis patients with and without neurocognitive deficit: a 3-year longitudinal study. Front Psychiatry 4:134
Gutierrez-Galve L, Chu EM, Leeson VC et al (2015) A longitudinal study of cortical changes and their cognitive correlates in patients followed up after first-episode psychosis. Psychol Med 45:205–216
Gutierrez-Galve L, Wheeler-Kingshott CA, Altmann DR et al (2010) Changes in the frontotemporal cortex and cognitive correlates in first-episode psychosis. Biol Psychiatry 68:51–60
Castro-Fornieles J, Parellada M, Gonzalez-Pinto A et al (2007) The child and adolescent first-episode psychosis study (CAFEPS): design and baseline results. Schizophr Res 91:226–237
Kaufman J, Birmaher B, Brent D et al (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988
Peralta V, Cuesta MJ (1994) Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiatry Res 53:31–40
Cannon-Spoor HE, Potkin SG, Wyatt RJ (1982) Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 8:470–484
Hollingshead A, Redlich F (1958) Social class and mental illness. Wiley, New York
Zabala A, Rapado M, Arango C et al (2010) Neuropsychological functioning in early-onset first-episode psychosis: comparison of diagnostic subgroups. Eur Arch Psychiatry Clin Neurosci 260:225–233
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Arango C (2012) Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry 69:16. doi:10.1001/archgenpsychiatry.2011.150
Chua SE, Cheung C, Cheung V et al (2007) Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res 89:12–21
Molina V, Sanz J, Villa R et al (2010) Voxel-based morphometry comparison between first episodes of psychosis with and without evolution to schizophrenia. Psychiatry Res 181:204–210
Watson DR, Anderson JM, Bai F et al (2012) A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav Brain Res 227:91–99
Reig S, Sanchez-Gonzalez J, Arango C et al (2009) Assessment of the increase in variability when combining volumetric data from different scanners. Hum Brain Mapp 30:355–368
Desco M, Hernandez JA, Santos A, Brammer M (2001) Multiresolution analysis in fMRI: sensitivity and specificity in the detection of brain activation. Hum Brain Mapp 14:16–27
Gogtay N, Sporn A, Clasen LS et al (2004) Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry 61:17
Gogtay N, Thompson PM (2010) Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn 72:6–15
Uda S, Matsui M, Tanaka C et al (2015) Normal development of human brain white matter from infancy to early adulthood: a diffusion tensor imaging study. Dev Neurosci 37:182–194
Mane A, Falcon C, Mateos JJ et al (2009) Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res 114:136–143
DeLisi LE (2007) The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull 34:312–321
McDonald C, Bullmore E, Sham P et al (2005) Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry 186:369–377
Woodberry KA, Giuliano AJ, Seidman LJ (2008) Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry 165:579–587
Ho B-C, Andreasen NC, Nopoulos P et al (2003) Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 60:585–594
Lappin JM, Morgan K, Morgan C et al (2006) Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res 83:145–153
Ho B-C, Andreasen NC, Ziebell S et al (2011) Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 68:128–137
Acknowledgements
This study was supported by a grant from the Carlos III Institute of Health, Cooperative Research Thematic Network (RETICS)-G03/032 and from the Spanish Ministry of Science and Innovation, CIBERSAM. Support was also given by SGR 489, and by the Alicia Koplowitz Foundation and S2010/BMD-2422 AGES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Rapado Castro was supported by a Sara Borrell Health Research Fellowship from the Institute of Health Carlos III, Spanish Ministry of Economy and Competitiveness, an Alicia Koplowitz Research Grant, an Alicia Koplowitz Short-Term Visiting Fellowship from the Alicia Koplowitz Foundation and an IiSGM Fellowship Award for Short-Term Placements from the Health Research Institute from the Hospital Gregorio Marañon (IiSGM) (Madrid, Spain). Dr. Gonzalez-Pinto has received grants and served as consultant, advisor or CME speaker for the following entities: Almirall, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Servier, Shering-Plough, Solvay, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), the Basque Government, the Stanley Medical Research Institute, and Wyeth. Dr. Graell was received honoraria or grants from Eli Lilly and Shire. Dr. Arango has been a consultant to or has received honoraria or grants from Abbot, AMGEN, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, Fundación Alicia Koplowitz, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Merck, Ministerio de Ciencia e Innovación, Ministerio de Sanidad, Ministerio de Economía y Competitividad, Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Schering Plough and Takeda.
Ethical Standards
The study was approved by the institutional review board at each clinical center. All parents or legal guardians gave written informed consent before the study began and all patients agreed to participate.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castro-Fornieles, J., Bargalló, N., Calvo, A. et al. Gray matter changes and cognitive predictors of 2-year follow-up abnormalities in early-onset first-episode psychosis. Eur Child Adolesc Psychiatry 27, 113–126 (2018). https://doi.org/10.1007/s00787-017-1013-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-1013-z