Abstract
Polar volcanoes harbor unique conditions of extreme temperature gradients capable of selecting different types of extremophiles. Deception Island is a marine stratovolcano located at Maritime Antarctica that is notable for its pronounced temperature gradients over very short distances, reaching values up to 100 °C in the fumaroles, and subzero temperatures next to the glaciers. Due to these characteristics, Deception can be considered an interesting analogue of extraterrestrial environments. Our main goal in this study was to isolate thermophilic and psychrophilic bacteria from sediments associated with fumaroles and glaciers from two geothermal sites in Deception Island, comprising temperatures between 0 and 98 °C, and to evaluate their survivability to desiccation and UV-C radiation. Our results revealed that culturable thermophiles and psychrophiles were recovered among the extreme temperature gradient in Deception volcano, which indicates that these extremophiles remain alive even when the conditions do not comprise their growth range. The viability of culturable psychrophiles in hyperthermophilic environments is still poorly understood and our work showed the importance of future studies about their survival strategies in high temperatures. Finally, the spore-forming thermophilic isolates which we found have displayed good survival to desiccation and UV-C irradiation, which suggests their potential to be further explored in astrobiological studies.
Similar content being viewed by others
Introduction
Polar volcanoes represent unique regions on Earth where all temperature-adapted bacteria (e.g., thermophiles, mesophiles, and psychrophiles) coexist and possibly interact in the same environment due to the pronounced temperature gradient (Amenábar et al. 2013). Deception Island is a marine stratovolcano located at Maritime Antarctica that is notable for its extreme steep temperature gradients, since its active fumaroles reach values of 100 °C, whereas half of the island is covered by glaciers (Rey et al. 1995; Herbold et al. 2014). These characteristics make Deception an interesting analogue of extraterrestrial environments, such as Mars’s extinct volcanoes and Enceladus’s cryovolcanoes (Soo et al. 2009; Sekine et al. 2015). The prominent temperature gradients over very short distances (e.g., a few meters) in Deception Island represent a unique opportunity to recover different culturable extremophiles.
Despite the development of new DNA sequencing technologies applying omics-based approaches in microbial ecology, cultivation studies are still relevant to assess the physiological properties of bacterial cells (Prakash et al. 2013). Cultures are important to provide the complete genomes and to test the hypotheses of metabolic potential and molecular adaptation that emerge from metagenomic data (Giovannoni and Stingl 2007). In addition, the isolation and recovery of living cells from extreme environments allow us to understand their survival strategies under simulated extraterrestrial conditions.
The microbial diversity of Deception Island has been studied over the last decades mainly through cultivation techniques. Llarch et al. (1997) isolated six thermophilic bacteria of the genus Bacillus from water and marine sediments associated with Deception fumaroles. Muñoz et al. (2011) have obtained the sequences of Geobacillus, Brevibacillus, and Thermus from culture enrichments of sediments associated with Deception fumaroles. Other works carried out in Deception describe the isolation of psychrophilic and psychrotolerant bacteria from lakes, marine sediments, and soils not associated with geothermal activity. Stanley and Rose (1967) identified bacteria of the genera Pseudomonas, Flavobacterium, and Achromobacter from five lakes of Deception. Carrión et al. (2011) isolated eight strains of marine sediments from the island, belonging to the genera Pseudomonas, Marinobacter, and Shewanella, and one of these strains was classified as a new species, proposed Pseudomonas deceptionensis. In a previous work, we reported the presence of several extremophiles-related sequences (psychrophiles, thermophiles, hyperthermophiles, and halophiles) among extreme temperature gradients in Deception (Bendia et al. 2018). However, since only 16S rRNA gene sequences were evaluated, it was not possible to know if these extremophiles were viable in those conditions.
To the best of our knowledge, no other work has investigated the viability of culturable extremophiles among the prominent temperature gradients in Deception Island, and focusing on their survival properties. Thereby, in this work, we aimed to isolate thermophilic and psychrophilic bacteria from sediments associated to fumaroles and glaciers from two geothermal sites in Deception Island, comprising temperatures between 0 and 98 °C with the purpose to evaluate their culturability through the extreme temperature gradients. In addition, we aimed to evaluate their potential application as models in astrobiological studies through desiccation and UV-C survival tests. Our results showed that isolates related to thermophilic and psychrophilic groups remain alive among the extreme temperature gradients in Deception Island volcano, even though the conditions do not comprise their growth range. In addition, our thermophilic isolates displayed a good resistance against desiccation and UV-C radiation, probably due to their spore-forming capacity. We proposed here the potential application of these thermophiles in future astrobiological studies.
Methodology
Sampling
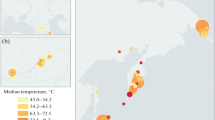
Sampling was performed during the XXXII Brazilian Antarctic Expedition (December 2013–January 2014) in Deception Island (62°58′ S, 60°39′ W) volcano, Antarctica, at the geothermally active sites of Fumarole Bay (FB) (62°58′02.7″ S, 60°42′ 36.4″ W) and Whalers Bay (WB) (62°58′45.1″ S, 60°33′27.3″ W) (Fig. 1a, b). In FB, we collected samples in fumaroles with temperatures of 98 °C (FBA) and 80 °C (FBB), and in a glacier (sediments below the glacier’s edge) with temperature below 0 °C (FBC). In WB, samples were collected in fumaroles with temperatures of 50 °C (WBA) and 10 °C (WBB), and in a glacier also with temperature below 0 °C (WBC) (Fig. 1b, c). Distances between fumaroles and glaciers at each site were approximately 15 m, and the WB and FB transects were approximately 10 km apart. All fumaroles were in the intertidal zone, with exception of fumarole of 80 °C from FB, which was in the subtidal zone (submerged at 50 cm depth in the water column). Samples were stored at 4 °C until arrival at the University of São Paulo, Brazil, in April 2014.
Sampling map. Location of Antarctic Peninsula (a) and Deception Island, with Fumarole Bay and Whalers Bay geothermal sites highlighted (b). The map in a was generated using ESRI ArcGIS software. The map in b was courtesy of British Antarctic Survey. Distribution of collected samples across environmental gradients at studied geothermal sites is described in c for Fumarole Bay and d for Whalers Bay. Values of in situ temperatures are represented in blue (glaciers) and orange (fumaroles). The arrow indicates the direction of low and high values of temperature
Estimation of total number of bacterial cells through quantitative PCR (qPCR)
We performed quantitative PCR (qPCR) to estimate the total number of bacterial cells per gram of sediment through 16S rRNA gene copies’ quantification. Reactions were carried out in triplicates in a total volume of 25 μL containing 12.5 μL of SYBR Green PCR Master Mix (Thermo Fisher Scientific, USA), 200 ng μL−1 of bovine serum albumin (Roche Diagnostics, Germany), and 0.2 μM of each primer (27F and 518R) (Lane 1991; Muyzer et al. 1993). Cycles began with the initial denaturation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, on StepOne Real-Time PCR System (Thermo Fisher Scientific, USA). The specificity of amplification products was analyzed by melting curves ranging between 60 and 95 °C. Standard curves were produced by serial dilutions of bacterial 16S rRNA gene from Escherichia coli, cloned in plasmids, and diluted from 10−3 to 10−8 per assay. We tested the presence of PCR inhibitors in our samples by combining 1 μL of positive controls (from 10−3 to 10−8 dilutions) with 1 μL of the extracted DNA in qPCR reactions. qPCR efficiency (E = 10 (1/−slope)) and linearity (R-squared value) were calculated from standard curves, and CT measurements were converted to number of copies per gram of sediment for each sample. For estimation of the total number of bacterial cells per gram of sediment, we considered the number of 3.8 copies of 16S rRNA gene per cell (Borrel et al. 2012).
Isolation procedures of extremophiles from environmental samples
To isolate psychrophiles and thermophiles from the extreme temperature gradient in Deception Island, we inoculated the samples in two culture media and two distinct growth temperatures. Enrichments were performed by adding 5 g of the sediment samples to 18 mL of Tryptic Soy Broth (Sigma-Aldrich) and Marine Broth (Difco) both diluted at 10% and with pH of 5.0. The dilution of the media at 10% was chosen with the intention of simulating the oligotrophic conditions found in the environment. Likewise, pH 5.0 was chosen according to the mean pH value measured in the environment. The enrichment cultures were incubated during 2 weeks at the temperatures of 4 and 60 °C for the recovery of culturable psychrophiles and thermophiles, respectively. After incubation, the enrichments were inoculated into their respective 10% solid media using serial dilutions (10−3–10−8) for colony isolation. For the solid media incubated at 60 °C, 8 g/L of Gelzan™ CM Gelrite® was added instead of agar with 0.5 g/L of CaCl2. We randomly selected three colonies of each culture for isolation procedure. A total of 147 colonies were successfully isolated and then selected for subsequent molecular analyses. Isolates were stored with 20% of glycerol at −80 °C and deposited in the Culture Collection at Oceanographic Institute, University of Sao Paulo.
BOX-PCR and molecular identification
To select only the distinct phylotypes for molecular identification, BOX-PCR was carried out with the extracted DNA from all obtained isolates. DNA was extracted directly from colonies by heating at 94 °C for 15 min. BOX-PCR was performed using a 0.75 μL of primer at 20 μM (5′-CTACGGCAAGGCGACGCTGACG-3′) (Versalovic et al. 1991), 12.5 μL of GoTaq® Hot Start Green Master Mix (Promega, USA), and 2 μl of DNA and 1.25 μl of DMSO (Sigma-Aldrich, USA). The PCR program consisted of the following reaction cycles: 94 °C for 7 min and 35 cycles at 94 °C for 1 min, 53 °C for 1 min and 72 °C for 8 min, and a final extension at 72° C for 15 min. The PCR products were observed with electrophoresis using a 2% agarose gel containing 4 μl of the SYBR Safe dye (Invitrogen, USA). The reference marker was 1 kb DNA Ladder Plus (Invitrogen, USA). The banding pattern of the isolates was grouped by similarity, and a dendrogram was constructed using the UPGMA method and Pearson’s coefficient in BioNumerics software v.5.10 (Applied Maths, Belgium). The distinct phylotypes were selected for the molecular identification with the 16S rRNA gene sequencing.
The sequencing of 16S rRNA gene was performed for the molecular identification of the isolates with distinct phylotypes. First, a PCR was performed using 27F and 1401R primers (Nübel et al. 1996; Hongoh et al. 2003), which consisted of: 0.2 μM of each primer, 12.5 μL of GoTaq® Hot Start Green Master Mix (Promega, USA), 2 μl of DNA, and 1.25 μl of DMSO (Sigma-Aldrich, USA). The thermocycler program started with a denaturation at 95° C for 3 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and final extension at 72 °C for 7 min. The amplicon was purified by the QIAquick Gel Extraction Kit (QIAGEN, USA) and quantified by Qubit 1.0 (Life Technologies, USA) with the Qubit® dsDNA HS Assay Kit (Life Technologies, USA). After purification, approximately 50 ng of the amplicons from each isolate were sent for sequencing by Sanger’s chain termination technique by Genomic Engenharia Molecular (São Paulo, Brazil). Sequencing reactions were performed using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) with the primers 27F and 1401R. The products were sequenced with the platform 3130xl from Applied Biosystems (USA).
The analysis of the sequences was initially performed using CodonCodeAligner Software (CodonCode Corporation, Dedham, MA, USA). Through this software, the sequences were checked for quality and treated, and contigs were formed from the overlap of the amplified sequences with the two primers employed. After obtaining the treated contigs, SILVA v128 (High-Quality Ribosomal RNA Databases) database was used to align the sequences, to identify the isolates and to construct the phylogenetic trees through the ARB software, using maximum-likelihood method (999 bootstraps) (Ludwig et al. 2004). All sequencing data were deposited in GenBank (National Center for Biotechnology Information Sequence Read Archives) under accession numbers between MH400077 and MH400151.
Survival of bacterial isolates to desiccation and UV-C radiation
We randomly selected three isolates of each genus that we identified through sequencing of 16S rRNA gene (with exception of the genera Thermus and Sphingomonas, since only one isolate was obtained from each one) to our desiccation survival tests. Thus, we selected a total of 10 and 12 isolates from 60 and 4 °C incubations, respectively. The desiccation assay was performed similar to the procedure described by Janning et al. (1994). First, isolates’ colonies were suspended in saline solution (0.9% NaCl) and their optical density was standardized to an absorbance between 0.5 and 1.5. A total volume of 10 μl from the cell suspensions of each isolate was deposited on the inner wall of 1.5 ml sterile microtubes and sealed in a chamber with silica gel. Silica gel was used to achieve a relative humidity below 5% inside the chamber. Non-desiccated, control samples were diluted and plated directly from the initial cell suspensions. After 9 days at room temperature (in the dark), samples were resuspended in 1 ml of saline solution (0.9% NaCl) and were diluted up to 10−5 (from the initial cell suspension) and plated for CFU counting using their respective solid media and growth temperature (60 °C or 4 °C). Survival of microorganisms was determined by the fraction N/N0 (N = number of viable cells recovered after desiccation; N0 = initial concentration of viable cells at the cell suspension).
Four thermophilic isolates (WBA3_3_AM, FBB1_2_AM, FBC2_1_AM, and WBB2_2_AM) were selected for UV-C survival tests. Preliminary assays showed that there are no significant differences between the survival of similar isolates (e.g., similar survival of all Anoxybacillus isolates). The UV-C survival tests were based on the previous work by Pulschen et al. (2015). We used a chamber equipped with a Philips TUV-20W low-pressure mercury tube lamp, with an emission peak at 253.7 nm. For the desiccation tests, plate-grown colonies were suspended in saline solution (0.9% NaCl) and standardized to an optical density between 0.5 and 1.5 for the irradiation tests. These suspensions were then serially diluted from \(10^{ - 1}\) to \(10^{ - 5}\), and 5 μl aliquots of each dilution, for each sample, were plated on solid media. The irradiations were then performed on these samples of cells and directly placed on the solid media surfaces, in successive fluences of 60 J/m2. The accumulated fluences used were: 0 J/m2 (control, non-irradiated), 60 J/m2, 120 J/m2, 180 J/m2, and 240 J/m2, measured with a Vilber Lourmat RMX-3 W radiometer equipped with a UV-C photocell (CX-254, Vilber Lourmat). After exposure, the irradiated plates were incubated overnight at 60 °C for the subsequent CFU counting. Survival of microorganisms was again determined by the fraction N/N0 (N = number of viable cells recovered at each fluence; N0 = initial concentration of viable cells at the non-irradiated drops).
Results
Estimated number of total bacterial cells through qPCR
Sediment from the hottest temperature (98 °C) (FBA) showed no amplification for bacterial 16S rRNA gene, probably because, in hyperthermophilic temperature, there is a dominance of archaeal groups in comparison to bacteria. Indeed, in a previous work, we detected only archaeal 16S rRNA sequences in FBA sample using Illumina sequencing technique (Bendia et al., 2018). The estimated number of bacterial cells per sediment gram on the other fumaroles was 3.38 × 104 in FBB, 6.33 × 104 in WBB, and 2.69 × 104 in WBA. In glaciers, the numbers were 2.01 × 104 and 1.51 × 107 for FBC and WBC, respectively.
Extremophiles isolated from environmental samples and molecular identification
After incubation in the solid media, we have randomly selected three colonies of each culture for the subsequent isolation procedure, preferably selecting colonies with distinct morphologies. The isolation of extremophilic bacteria resulted in 67 isolates cultivated at 60 °C and 82 isolates at 4 °C (Table 1). After the selection of distinct phylotypes through BOX-PCR, 54 isolates at 60 °C and 45 isolates at 4 °C were selected for molecular identification (Supplementary Figs. 1 and 2). However, 4 isolates at 60 °C and 16 isolates at 4 °C could not have their rRNA 16S gene amplified under the analyzed conditions. Phylogenetic analysis using maximum-likelihood method for 16S rRNA gene showed six main clades for thermophiles and five clades for psychrophiles (Supplementary Figs. 3 and 4). These clades include thermophilic members of the genera Geobacillus, Brevibacillus, Anoxybacillus, Thermus, and Bacillales order, and psychrophilic members of Arthrobacter, Psychrobacter, Flavobacterium, Pseudomonas, and Sphingomonas, accordingly with SILVA database v128.
The majority of isolates grown at 60 °C were assigned to the genus Geobacillus (56%), 22% were related to Brevibacillus thermoruber (11 isolates), 14% to Anoxybacillus kestanbolensis (7 isolates), 6% to the order Bacillales, and 2% were assigned as Thermus thermophilus (Fig. 4a). Isolates grown at 4 °C were classified as Arthrobacter (23%), Psychrobacter (11.5%), Flavobacterium (38.5%), Pseudomonas (23%), and Sphingomonas (4%) (Fig. 2) (Table 1).
Relative abundances of the taxonomic groups assigned to the isolates grown at 60 °C (67 isolates) (a) and 4 °C (80 isolates) (b) at the maximum classification level. Environmental temperatures of each sample are represented. Sequences were assigned with 97% similarity against the SILVA database v128
Geobacillus-related isolates were recovered along all environmental temperature gradients in both Marine Agar and TSA media incubated at 60 °C. Thermus thermophilus was obtained only from FB sample (80 °C) grown in Marine Agar at 60 °C. Brevibacillus thermoruber and Anoxybacillus kestanbolensis were recovered only from Marine Agar from sediments with environmental temperatures below 50 °C. Isolates related to Bacillales order were recovered from WB glacier with Marine Agar medium (Fig. 2a). When aligned with NCBI database using BLAST searches, sequences related to order Bacillales (WBC2_3_AM, WBC3_2_AM, and WBC3_1_AM) showed 97%, 99%, and 99% of identity with Caenibacillus caldisaponilyticus (NR_149766.1), respectively.
In contrast, none of our isolates from 4 °C incubations was recovered along the complete temperature gradient. We recovered from fumaroles, isolates related to Arthrobacter, Flavobacterium, Sphingomonas, Psychrobacter, and Pseudomonas. Furthermore, Sphingomonas was only isolated from FBB sample (80 °C) using Marine Agar medium. In our glacier samples (< 0 °C), all isolates were related to Pseudomonas, Arthrobacter, and Flavobacterium. Moreover, Arthrobacter was mainly obtained from Whalers Bay fumaroles. Surprisingly, we also isolated Arthrobacter from the hottest fumarole (FBA, 98 °C) (Fig. 2b, Table 1). The 16S rRNA sequence of Arthrobacter from the hottest fumarole showed to be more similar to Arthrobacter from fumaroles > 50 °C in comparison with Arthrobacter from glaciers (Supplementary Fig. 5).
Survival of bacterial isolates to desiccation and UV-C radiation
Thermophilic isolates showed higher desiccation survival rates when compared to the psychrophiles (Fig. 3). In general, the selected thermophilic isolates displayed good desiccation survival, losing 0.5–1.5 logs in viability after 9 days of desiccation. In contrast, all selected psychrophilic isolates lost 3–4 logs of viability. Since thermophilic isolates displayed best survival during desiccation, five representatives were selected for UV-C irradiation assays (Fig. 4). Brevibacillus thermoruber and Anoxybacillus kestanbolensis displayed the best survival, capable of enduring up to 240 J/m2 of UV-C irradiation before losing 1 log of viability, whereas the Gram-negative, non-sporulating Thermus thermophilus strain showed to be the most sensitive to UV-C radiation among the tested isolates, losing almost 2 logs of viability upon 180 J/m2.
Discussion
Extremophiles from geothermal habitats have been studied over the last decades; however, a few studies focused on understanding the viability of culturable bacteria through steep temperature gradients usually found in the polar volcanoes. Deception Island provides a unique opportunity to study temperature-adapted bacteria due to the closeness between fumaroles and glaciers. Using cultivation techniques to isolate thermophiles and psychrophiles from Deception fumaroles and glaciers, we obtained these culturable extremophiles across all the temperature gradients. These results suggest that polar volcanoes such as Deception allow the coexistence and a possible interaction between thermophiles and psychrophiles. Furthermore, our results suggest that our spore-forming thermophilic isolates presented a good survival towards desiccation and UV-C radiation, and could be applied as future models for astrobiological studies.
In our study, we obtained thermophiles from the genera Geobacillus, Brevibacillus, Anoxybacillus, Thermus, and Bacillales order. These groups were previously described in Deception sediments associated with fumaroles using different growth media (Llarch et al. 1997; Muñoz et al. 2011). However, in our work, we recovered these thermophiles from both fumaroles and glaciers, with environmental temperatures between 80 and 0 °C (Fig. 4). Several works suggested that microorganisms were often detected in environmental conditions that do not comprise their growth range (Hubert et al. 2009). A previous study has shown that thermophilic microorganisms can be trapped in glaciers by process of accretion and then be preserved for long periods (Bulat et al. 2004). Hubert et al. (2009) have detected inactive thermophilic bacteria of Firmicutes phylum in cold Arctic sediments that were rapidly activated when submitted to their optimal growth conditions. Rahman et al. (2004) described thermophilic bacteria in cool soil environments of Ireland and suggested the autochthonous nature of the thermophilic bacteria inhabiting cold ecosystems. Dormant thermophilic cells can thrive in hostile environments through sporulation, explaining the presence of these viable microorganisms in inhospitably cold habitats (Hubert et al. 2009). In fact, the majority of our thermophilic isolates (with exception of Thermus thermophilus) are spore-forming bacteria (Manachini et al. 1985; Dulger et al. 2004; Zeigler 2014), which can help to explain their culturability in the Deception glaciers.
We also isolated psychrophiles related to Arthrobacter, Psychrobacter, Flavobacterium, Pseudomonas, and Sphingomonas genera in both Deception fumaroles and glaciers. Pseudomonas and Flavobacterium were previously isolated from Deception samples associated with lakes and cold marine sediments (Stanley and Rose 1967; Carrión et al. 2011). Arthrobacter, Psychrobacter, and Sphingomonas have been described in several Antarctic ecosystems, such as soils, marine sediments, and sea ice (e.g., Bowman et al. 1997; Turkiewicz et al. 1999; Reddy et al. 2000; Prabagaran et al. 2007; Baraniecki et al. 2002; Dsouza et al. 2015), showing their cosmopolitan nature in cold ecosystems. Culturable cells related to psychrophilic groups are poorly described in hot environments and the majority of studies have detected these microorganisms through culture-independent genomic methods, which do not allow us to know if they were in fact alive in those conditions. For example, Kaur et al. (2018) obtained sequences related to Arthrobacter from hot spring soil in India with temperatures from 50 to 90 °C through metagenomics. Psychrobacter sequences were obtained from geothermal water samples in Iceland with temperatures around 40 °C through pyrosequencing of 16S rRNA gene (Palinska et al. 2018). Sphingomonas members, which could be psychrophilic or mesophilic chemoorganotrophs, were detected in deep-sea hydrothermal field of the Suiyo Seamount using clone library technique (Kato et al. 2009).
Groups with known psychrophilic members.
The psychrophilic groups that we found in Deception have usually a maximum growth temperature below 35 °C (Bakermans and Nealson 2004; Lauro et al. 2011; Cavicchioli 2016) and, to the best of our knowledge, no other work has described their viability in hyperthermophilic temperatures. One strain of Psychrobacter piscatorii has been isolated from deep-sea hydrothermal vents on the East Pacific Rise and draft genomic analyses have shown the presence of more catalase genes in comparison to other Psychrobacter genomes (Zhou et al. 2016). The presence of these catalase genes may favor Psychrobacter adaptation to oxidative environments, as those found in geothermal systems with hydrogen sulfide emissions. The additional genes’ content of some psychrophilic strains as those described by Zhou et al. (2016) probably helps the psychrophile adaptation in geothermal environments and could explain their culturability in our fumaroles samples.
We also tested our obtained isolates to desiccation and UV-C resistance, seeking to investigate their potential as astrobiological models. In fact, samples from extreme environments (as Antarctica and Atacama as ideal) have been constantly explored in the search of microbes that could be further studied in the astrobiology context (Musilova et al. 2015; Pulschen et al. 2015). We observed a clear distinction from the retrieved thermophilic isolates and psychrophilic isolates to desiccation survival (Fig. 3), which we attribute to the fact that, excluding T. thermophilus, all thermophilic isolates tested are spore-forming bacteria (Manachini et al. 1985; Dulger et al. 2004; Zeigler 2014). Therefore, we decided to explore further such spores’ resistance, exposing them to UV-C irradiation. The UV-C radiation is more energetic and bactericidal that longer UV wavelengths as UV-B and UV-A. Although not present on Earth, it composes a significant proportion of UV spectra on the Martian surface, due to the rarified atmosphere of the planet (Cockell et al. 2000), therefore, having significant importance to astrobiology studies (Paulino-Lima et al. 2013; Pulschen et al. 2015). We observed that B. thermoruber and A. kestanbolensis displayed the best survival, scoring a good survival after UV-C irradiation, with a D10 of 200 J/m2. For comparative purposes, the described D10 of Escherichia coli to UV-C radiation is 30 J/m2 (Paulino-Lima et al. 2013). As expected, Thermus thermophilus displayed the lowest survival to UV-C (Fig. 4), compared to the other tested thermophilic organisms.
Bacterial spores are well-established models for astrobiology, due to their resistance to multiple factors, including desiccation and UV irradiation. Spores of Bacillus pumilus and Bacillus subtilis, for example, have already been exposed and tested for their survival under true space conditions (Horneck et al. 2012; Panitz et al. 2015). Recently, Khodadad et al. (2017) directly exposed desiccated spores of B. pumilus to the stratosphere (above 30 km of altitude). Remarkably, after 4 h of direct exposure, viable spores could still be retrieved. Due to such high resistance of bacterial spores, they are also studied in the context of planetary protection (Schuerger et al. 2003; Khodadad et al. 2017).
Conclusion
Using cultivation techniques, we obtained psychrophilic and thermophilic isolates among all the pronounced temperature gradients (from 0 to 98 °C) on Deception Island, which indicates that the extremophilic cells remain viable even when the conditions do not support their metabolic activity. Although the previous studies have been isolated thermophiles from cold ecosystems, the recovery of psychrophiles in hyperthermophilic environments is still poorly understood and our work suggests the importance of studies about their survival strategies in high temperatures. Finally, our thermophilic isolates, especially those belonging to spore-forming genera, have displayed resistance towards desiccation and UV-C irradiation. These initial data suggest that such thermophilic spores have potential to be further explored in astrobiological studies.
References
Amenábar MJ, Flores PA, Pugin B et al (2013) Archaeal diversity from hydrothermal systems of Deception Island, Antarctica. Polar Biol 36:373–380
Bakermans C, Nealson KH (2004) Relationship of critical temperature to macromolecular synthesis and growth yield in Psychrobacter cryopegella. J Bacteriol. https://doi.org/10.1128/JB.186.8.2340-2345.2004
Baraniecki CA, Aislabie J, Foght JM (2002) Characterization of Sphingomonas sp. Ant 17, an aromatic hydrocarbon-degrading bacterium isolated from Antarctic soil. Microb Ecol. https://doi.org/10.1007/s00248-001-1019-3
Bendia AG, Signori CN, Franco DC et al (2018) A mosaic of geothermal and marine features shapes microbial community structure on Deception Island volcano. Antarctica. Front Microbiol 9:899. https://doi.org/10.3389/fmicb.2018.00899
Borrel G, Lehours AC, Crouzet O et al (2012) Stratification of Archaea in the deep sediments of a freshwater meromictic lake: vertical shift from methanogenic to uncultured Archaeal lineages. PLoS One. https://doi.org/10.1371/journal.pone.0043346
Bowman JP, Nichols DS, McMeekin TA (1997) Psychrobacter glacincola sp. nov., a halotolerant, psychrophilic bacterium isolated from Antarctic Sea ice. Syst Appl Microbiol. https://doi.org/10.1016/s0723-2020(97)80067-7
Bulat SA, Alekhina IA, Blot M et al (2004) DNA signature of thermophilic bacteria from the aged accretion ice of Lake Vostok, Antarctica: implications for searching for life in extreme icy environments. Int J Astrobiol. https://doi.org/10.1017/s1473550404001879
Carrión O, Miñana-Galbis D, Montes MJ, Mercadé E (2011) Pseudomonas deceptionensis sp. nov., a psychrotolerant bacterium from the antarctic. Int J Syst Evol Microbiol 61:2401–2405
Cavicchioli R (2016) On the concept of a psychrophile. ISME J 10:793–795
Cockell CS, Catling DC, Davis WL et al (2000) The ultraviolet environment of Mars: biological implications past, present, and future. Icarus. https://doi.org/10.1006/icar.2000.6393
Dsouza M, Taylor MW, Turner SJ, Aislabie J (2015) Genomic and phenotypic insights into the ecology of Arthrobacter from Antarctic soils. BMC Genom. https://doi.org/10.1186/s12864-015-1220-2
Dulger S, Demirbag Z, Belduz AO (2004) Anoxybacillus ayderensis sp. nov. and Anoxybacillus kestanbolensis sp. nov. Int J Syst Evol Microbiol. https://doi.org/10.1099/ijs.0.02863-0
Giovannoni S, Stingl U (2007) The importance of culturing bacterioplankton in the “omics” age. Nat Rev Microbiol 5:820–826
Herbold CW, McDonald I, Cary C (2014) Microbial Ecology of Geothermal Habitats in Antarctica. In: Cowan D (ed) Antarctic Terrestrial Microbiology. Springer, Berlin Heidelberg, pp 181–215
Hongoh Y, Ohkuma M, Kudo T (2003) Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae). FEMS Microbiol Ecol. https://doi.org/10.1016/s0168-6496(03)00026-6
Horneck G, Moeller R, Cadet J et al (2012) Resistance of bacterial endospores to outer space for planetary protection purposes—experiment PROTECT of the EXPOSE-E mission. Astrobiology. https://doi.org/10.1089/ast.2011.0737
Hubert C, Loy A, Nickel M et al (2009) A constant flux of diverse thermophilic bacteria into the cold arctic seabed. Science. https://doi.org/10.1126/science.1174012
Janning B, in’t Veld PH, Notermans S, Krämer J (1994) Resistance of bacterial strains to dry conditions: use of anhydrous silica gel in a desiccation model system. J Appl Bacteriol. https://doi.org/10.1111/j.1365-2672.1994.tb03080.x
Kato S, Hara K, Kasai H et al (2009) Spatial distribution, diversity and composition of bacterial communities in sub-seafloor fluids at a deep-sea hydrothermal field of the Suiyo Seamount. Deep Sea Res Part I Oceanogr Res Pap. https://doi.org/10.1016/j.dsr.2009.05.004
Kaur R, Rajesh C, Sharma R et al (2018) Metagenomic investigation of bacterial diversity of hot spring soil from Manikaran, Himachal Pradesh, India. Ecol Genet Genom. https://doi.org/10.1016/j.egg.2017.11.003
Khodadad CL, Wong GM, James LM et al (2017) Stratosphere conditions inactivate bacterial endospores from a Mars spacecraft assembly facility. Astrobiology. https://doi.org/10.1089/ast.2016.1549
Lane DJ (1991) 16S/23S rRNA Sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, pp 115–175
Lauro FM, Allen MA, Wilkins D et al (2011) Psychrophiles: genetics, genomics, evolution. Extremophiles Handbook. Springer, Tokyo, pp 865–890
Llarch À, Logan NA, Castellví J et al (1997) Isolation and characterization of thermophilic Bacillus spp. from geothermal environments on Deception Island, South Shetland Archipelago. Microb Ecol 34:58–65
Ludwig W, Strunk O, Westram R et al (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Manachini PL, Fortina MG, Parini C, Craveri R (1985) Bacillus thermoruber sp. nov., nom. rev., a red-pigmented thermophilic bacterium. Int J Syst Evol Microbiol 35:493–496
Muñoz PA, Flores PA, Boehmwald FA, Blamey JM (2011) Thermophilic bacteria present in a sample from Fumarole Bay, Deception Island. Antarct Sci 23:549–555
Musilova M, Wright G, Ward JM, Dartnell LR (2015) Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance. Astrobiology. https://doi.org/10.1089/ast.2014.1278
Muyzer G, Uitterlinden AG, Uitterlinden AG, De Waal EC (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nübel U, Engelen B, Felsre A et al (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol. https://doi.org/10.1128/jb.178.19.5636-5643.1996
Palinska KA, Vogt JC, Surosz W (2018) Biodiversity analysis of the unique geothermal microbial ecosystem of the Blue Lagoon (Iceland) using next-generation sequencing (NGS). Hydrobiologia. https://doi.org/10.1007/s10750-017-3349-2
Panitz C, Horneck G, Rabbow E et al (2015) The SPORES experiment of the EXPOSE-R mission: Bacillus subtilis spores in artificial meteorites. Int J Astrobiol. https://doi.org/10.1017/S1473550414000251
Paulino-Lima IG, Azua-Bustos A, Vicuña R et al (2013) Isolation of UVC-Tolerant bacteria from the hyperarid Atacama Desert, Chile. Microb Ecol. https://doi.org/10.1007/s00248-012-0121-z
Prabagaran SR, Manorama R, Delille D, Shivaji S (2007) Predominance of Roseobacter, Sulfitobacter, Glaciecola and Psychrobacter in seawater collected off Ushuaia, Argentina, Sub-Antarctica. FEMS Microbiol Ecol 59:342–355
Prakash O, Shouche Y, Jangid K, Kostka JE (2013) Microbial cultivation and the role of microbial resource centers in the omics era. Appl Microbiol Biotechnol 97(1):51–62
Pulschen AA, Rodrigues F, Duarte RTD et al (2015) UV-resistant yeasts isolated from a high-altitude volcanic area on the Atacama Desert as eukaryotic models for astrobiology. Microbiologyopen. https://doi.org/10.1002/mbo3.262
Rahman TJ, Marchant R, Banat IM (2004) Distribution and molecular investigation of highly thermophilic bacteria associated with cool soil environments. Biochem Soc Trans. https://doi.org/10.1042/BST0320209
Reddy GSN, Aggarwal RK, Matsumoto GI, Shivaji S (2000) Arthrobacter flavus sp. nov., a psychrophilic bacterium isolated from a pond in McMurdo Dry Valley, Antarctica. Int J Syst Evol Microbiol. https://doi.org/10.1099/00207713-50-4-1553
Rey J, Somoza L, Martínez-Frías J (1995) Tectonic, volcanic, and hydrothermal event sequence on Deception Island (Antarctica). Geo-Marine Lett. https://doi.org/10.1007/BF01204491
Schuerger AC, Mancinelli RL, Kern RG et al (2003) Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated martian environments: implications for the forward contamination of Mars. Icarus 165:253–276
Sekine Y, Shibuya T, Postberg F et al (2015) High-temperature water–rock interactions and hydrothermal environments in the chondrite-like core of Enceladus. Nat Commun 6:8604. https://doi.org/10.1038/ncomms9604
Soo RM, Wood SA, Grzymski JJ et al (2009) Microbial biodiversity of thermophilic communities in hot mineral soils of Tramway Ridge, Mount Erebus, Antarctica. Environ Microbiol 11:715–728
Stanley SO, Rose AH (1967) Bacteria and yeasts from lakes on Deception Island. Philos Trans R Soc London B Biol Sci 252:199–207
Turkiewicz M, Gromek E, Kalinowska H, Zielińska M (1999) Biosynthesis and properties of an extracellular metalloprotease from the Antarctic marine bacterium Sphingomonas paucimobilis. Prog Ind Microbiol. https://doi.org/10.1016/S0079-6352(99)80097-6
Versalovic J, Koeuth T, Lupski JR (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831
Zeigler DR (2014) The Geobacillus paradox: why is a thermophilic bacterial genus so prevalent on a mesophilic planet? Microbiology (UK). https://doi.org/10.1099/mic.0.071696-0
Zhou M, Dong B, Liu Q (2016) Draft Genome Sequence of Psychrobacter piscatorii Strain LQ58, a Psychrotolerant Bacterium Isolated from a Deep-Sea Hydrothermal Vent. Genome Announc. https://doi.org/10.1128/genomeA.00044-16
Acknowledgements
We thank the captain and the crew of the research polar vessel Almirante Maximiano, Dr. Wânia Duleba, and Dr. Antônio Carlos Rocha Campos for their support in sampling. We are very thankful to LECOM’s research team and Rosa C. Gamba for their scientific support.
Funding
This study was part of the projects Microsfera (CNPq 407816/2013-5), INCT-Criosfera (CNPq 028306/2009), and Universal (CNPq 424367/2016-5), supported by the Brazilian National Council of Technological and Scientific Development (CNPq) and the Brazilian Antarctic Program (ProAntar). This study was also part of the project “Probing the Martian Environment with Experimental Simulations and Terrestrial Analogues” (project G-1709-20205), supported by Serrapilheira Institute. The São Paulo Research Foundation—FAPESP—supported the following fellowships: AB Doctorate’s fellowship (2012/23241-0) and RD Post Doc fellowship (2012/11037-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by A. Oren.
Electronic supplementary material
Below is the link to the electronic supplementary material.
792_2018_1048_MOESM1_ESM.pdf
Supplementary Fig. 1 Dendrogram showing the fingerprinting profiles generated by the BOX-PCR of 67 isolates grown at 60 °C, which were grouped into 54 different phylotypes. For these analyses, Pearson’s coefficient and the UPGMA method for clusters formation were used
792_2018_1048_MOESM2_ESM.pdf
Supplementary Fig. 2 Dendrogram showing the fingerprinting profiles generated by the BOX-PCR of 80 isolates grown at 4 °C, which were grouped into 45 different phylotypes. For these analyses, Pearson’s coefficient and the UPGMA method for clusters formation were used
792_2018_1048_MOESM3_ESM.jpeg
Supplementary Fig. 3 Phylogenetic tree relating the isolates grown at 60 °C with its similar taxonomic groups selected in the SILVA database v128 (> 97% similarity). The method used to construct the trees was maximum likelihood (with 999 bootstraps)
792_2018_1048_MOESM4_ESM.jpeg
Supplementary Fig. 4 Phylogenetic tree relating the isolates grown at 4 °C with its similar taxonomic groups selected in the SILVA database v128 (> 97% similarity). The method used to construct the trees was maximum likelihood (with 999 bootstraps)
792_2018_1048_MOESM5_ESM.pdf
Supplementary Fig. 5 Alignment of 16S rRNA gene between Arthrobacter isolates from hottest fumarole (FBA1_3_TSA) and Arthrobacter isolates from coldest samples. Y axis represents the alignment similarity (%) and X axis the region of 16S rRNA gene in base pairs (bp)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bendia, A.G., Araujo, G.G., Pulschen, A.A. et al. Surviving in hot and cold: psychrophiles and thermophiles from Deception Island volcano, Antarctica. Extremophiles 22, 917–929 (2018). https://doi.org/10.1007/s00792-018-1048-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-018-1048-1