Abstract
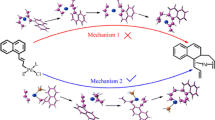
Density functional theory (DFT) was used to investigate the nickel- or nickel(0)/zinc- catalyzed decarbonylative addition of phthalic anhydrides to alkynes. All intermediates and transition states were optimized completely at the B3LYP/6-31+G(d,p) level. Calculated results indicated that the decarbonylative addition of phthalic anhydrides to alkynes was exergonic, and the total free energy released was −87.6 kJ mol−1. In the five-coordinated complexes M4a and M4b, the insertion reaction of alkynes into the Ni-C bond occurred prior to that into the Ni-O bond. The nickel(0)/zinc-catalyzed decarbonylative addition was much more dominant than the nickel-catalyzed one in whole catalytic decarbonylative addition. The reaction channel CA→M1'→T1'→M2'→T2'→M3a'→M4a'→T3a1'→M5a1' →T4a1'→M6a'→P was the most favorable among all reaction pathways of the nickel- or nickel(0)/zinc- catalyzed decarbonylative addition of phthalic anhydrides to alkynes. And the alkyne insertion reaction was the rate-determining step for this channel. The additive ZnCl2 had a significant effect, and it might change greatly the electron and geometry structures of those intermediates and transition states. On the whole, the solvent effect decreased the free energy barriers.

DFT study suggests that NiL4/ZnCl2 (L=PMe3) has higher catalysis than NiL4 in the synthesis of isocoumarin from phthalic anhydrides and alkynes.








Similar content being viewed by others
References
Agata N, Nogi H, Milhollen M, Kharbanda S, Kufe D (2004) 2-(8-hydroxy-6-methoxy-1-oxo -1H-2-benzopyran-3-yl)propionic acid, a small molecule isocoumarin, potentiates dexamethasone-induced apoptosis of human multiple myeloma mells. Cancer Res 64:8512–8516
Pochet L, Frédérick R, Masereel B (2004) Coumarin and isocoumarin as serine protease inhibitors. Curr Pharm Design 10:3781–3796
Fukuyama T, Higashibeppu Y, Yamaura R, Ryu I (2007) Complex α–pyrones synthesized by a gold-catalyzed coupling reaction. Org Lett 9:587–589
Luo T, Schreiber SL (2007) Complex α-pyrones synthesized by a gold-catalyzed coupling reaction. Angew Chem Int Ed 46:8250–8253
Woo JCS, Walker SD, Faul MM (2007) Preparation and decarboxylative rearrangement of (Z)-enyne esters. Tetrahedron Lett 48:5679–5682
Kajita Y, Kurahashi T, Matsubara S (2008) Nickel-catalyzed decarbonylative addition of anhydrides to alkynes. J Am Chem Soc 130:17226–17227
Fischer R, Walther D, Kempe R, Sieler J, Schönecker B (1993) Metallacyclische acyl-carboxylate des nickels: Reaktivität der acylgruppe und synthesepotential bei kreuzkopplungsreaktionen mit alkylhalogeniden. J Organomet Chem 447:131–136
O’Brien EM, Bercot EA, Rovis T (2003) Decarbonylative Cross-coupling of cyclic anhydrides: introducing stereochemistry at an sp3 carbon in the cross-coupling event. J Am Chem Soc 125:10498–10499
Johnson JB, Bercot EA, Rowley JM, Coates GW, Rovis T (2007) Ligand-dependent catalytic cycle and role of styrene in nickel-catalyzed anhydride cross-coupling: evidence for turnover-limiting reductive elimination. J Am Chem Soc 129:2718–2725
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven JT, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PW, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision D.02. Gaussian, Inc, Wallingford
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Flükiger P, Lüthi HP, Portmann S, Weber J (2000–2002) MOLEKEL 4.3 Swiss Center for Scientific Computing, Manno. Switzerland
Portmann S, Lüthi HP (2000) An interactive molecular graphics tool. Chimia 54:766–770
Carpenter JE, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct (THEOCHEM) 169:41–50
Foster JP, Weinhold F (1980) Natural hybridorbitals. J Am Chem Soc 102:7211–7218
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phy 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) NBO 5.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, WI
Marten B, Kim K, Cortis C, Friesner RA, Murphy RB, Ringnalda MN, Sitkoff D, Honig B (1996) New model for calculation of solvation free energies: correction of self-consistent reaction field continuum dielectric theory for short range hydrogen-bonding effects. J Phys Chem 100:11775–11788
Friesner RA, Murphy RB, Beachy MD, Ringnalda MN, Pollard WT, Dunietz BD, Cao YX (1999) Correlated ab initio electronic structure calculations for large molecules. J Phys Chem A 103:1913–1928
Miertus S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–245
Bader RFW (1990) Atoms in molecules, a quantum theory; international series of monographs in chemistry, vol 22. Oxford University Press, Oxford
Biegler-König F, Schönbohm J, Derdau R, Bayles D, Bader RFW (2002) AIM 2000. Version 2.0, McMaster University
Acknowledgments
This work was supported by the Key Project of Science and Technology of the Ministry of Education, P. R. (grant No. 104263), Natural Science Foundation of Chongqing City, P. R. (grant No. CSTC-2004BA4024). Contract grant sponsor: Science and Technology of the Ministry of Education, People’s Republic of China (NO: 104263); Natural Science Foundation of Chongqing City, P. R. (No: CSTC-2004BA4024).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Meng, Q., Li, M. Nickel/zinc-catalyzed decarbonylative addition of anhydrides to alkynes: A DFT study. J Mol Model 19, 4545–4554 (2013). https://doi.org/10.1007/s00894-013-1968-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1968-8