Abstract
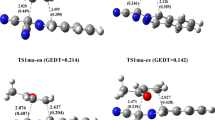
The synthesis of 3-acyltetramic acids by C-acylation of pyrrolidine-2,4-diones was studied by density functional theory (DFT). DFT was applied to the mycotoxin tenuazonic acid (TeA), an important representative of these bioactive natural compounds. Lewis acid mediated C-acylation in combination with previous pH-neutral domino N-acylation–Wittig cyclization can be used for the efficient preparation of 3-acyltetramic acids. Nevertheless, quite harsh conditions are still required to carry out this synthetic step, leading to unwanted isomerization of stereogenic centers in some cases. In the presented study, the reaction pathway for the C-acetylation of (5S,6S-5-s-butylpyrrolidine-2,4-dione was studied in terms of mechanism, solvent effects, and Lewis acid activation, in order to obtain an appropriate theoretical model for further investigations. Crucial steps were identified that showed rather high activation barriers and rationalized previously reported experimental discoveries. After in silico optimization, aluminum chlorides were found to be promising Lewis acids that promote the C-acylation of pyrrolidine-2,4-diones, whereas calculations performed in various organic solvents showed that the solvent had only a minor effect on the energy profiles of the considered mechanisms. This clearly indicates that further synthetic studies should focus on the Lewis-acidic mediator rather than other reaction parameters. Additionally, given the results obtained for different reaction routes, the stereochemistry of this C-acylation is discussed. It is assumed that the formation of Z-configured TeA is favored, in good agreement with our previous studies.

Lewis acid mediated synthesis of tetramic acids starting from pyrrolidine-2,4-diones (shown for tenuazonic acid).
















Similar content being viewed by others
References
Schobert R, Schlenk A (2008) Tetramic and tetronic acids: an update on new derivatives and biological aspects. Bioorg Med Chem 16:4203–4221
Royles BJL (1995) Naturally occurring tetramic acids: structure, isolation, and synthesis. Chem Rev 95:1981–2001
Rosett T, Sankhala RH, Stickings CE, Taylor MEU, Thomas R (1957) Studies in the biochemistry of micro-organisms—metabolites of Alternaria-tenuis auct—culture filtrate products. Biochem J 67:390–400
Stickings CE (1959) Studies in the biochemistry of micro-organisms—metabolites of Alternaria tenuis auct—the structure of tenuazonic acid. Biochem J 72:332–340
Meronuck RA, Steele JA, Mirocha CJ, Christensen CM (1972) Tenuazonic acid, a toxin produced by Alternaria alternata. Appl Microbiol 23:613–617
Aoki S, Higuchi K, Ye Y, Satari R, Kobayashi M (2000) Melophlins A and B, novel tetramic acids reversing the phenotype of RAS-transformed cells, from the marine sponge Melophlus sarassinorum. Tetrahedron 56:1833–1836
Lin Z-J, Lu Z-Y, Zhu T-J, Fang Y-C, Gu Q-Q, Zhu W-M (2008) Penicillenols from Penicillium sp. GQ-7, an endophytic fungus associated with Aegiceras corniculatum. Chem Pharm Bull 56:217–221
Höltzel A, Gänzle MG, Nicholson GJ, Hammes WP, Jung G (2000) The first low molecular weight antibiotic from lactic acid bacteria: reutericyclin, a new tetramic acid. Angew Chem Int Ed 39:2766–2768
Lacey RN (1954) Derivatives of acetoacetic acid. α-Acetyltetramic acids. J Chem Soc 850–854.
Kozikowski AP, Greco MN, Springer JP (1984) Total synthesis of the unique mycotoxin α-cyclopiazonic acid (αCA): an unusual dimethylzinc mediated replacement of a phenylthio substituent by a methyl group and a contrathermodynamic Raney nickel desulfurization reaction. J Am Chem Soc 106:6873–6874
Harris SA, Fisher LV, Folkers K (1965) The synthesis of tenuazonic and congeneric tetramic acids. J Med Chem 8:478–482
Böhme R, Jung G, Breitmaier E (2005) Synthesis of the antibiotic (R)-reutericyclin via Dieckmann condensation. Helv Chim Acta 88:2837–2841
Jones RCF, Begley MJ, Peterson GE, Sumaria S (1990) Acylation of pyrrolidine-2,4-diones: a synthesis of 3-acyltetramic acids. X-ray molecular structure of 3-[1-(difluoroboryloxy)ethylidene]-5-isopropyl-1-methyl-pyrrolidine-2,4-dione. J Chem Soc Perkin Trans 1:1959–1968
Schobert R, Dietrich M, Mullen G, Urbina-Gonzalez J-M (2006) Phosphorus ylide based functionalizations of tetronic and tetramic acids. Synthesis 2006:3902–3914
Biersack B, Diestel R, Jagusch C, Rapp G, Sasse F, Schobert R (2008) First syntheses of melophlins P, Q, and R, and effects of melophlins on the growth of microorganisms and tumor cells. Chem Biodivers 5:2423–2430
Schobert R, Jagusch C, Melanophy C, Mullen G (2004) Synthesis and reactions of polymer-bound Ph3P=C=C=O: a quick route to tenuazonic acid and other optically pure 5-substituted tetramates. Org Biomol Chem 2:3524–3529
Lohrey L, Marschik S, Cramer B, Humpf H-U (2012) Large-scale synthesis of isotopically labeled 13C2-tenuazonic acid and development of a rapid HPLC-MS/MS method for the analysis of tenuazonic acid in tomato and pepper products. J Agric Food Chem 61:114–120
Stewart JP (2007) Optimization of parameters for semiempirical methods. V: Modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, London
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. A basis set for correlated wave functions. J Chem Phys 72:650–654
Becke AD (1993) Density-functional thermochemistry. The role of exact exchange. J Chem Phys 98:5648–5652
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian Inc., Wallingford
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094
Fukui K (1981) The path of chemical reactions—the IRC approach. Acc Chem Res 14:363–368. doi:10.1021/ar00072a001
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CA, Weinhold F (2001) NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin, Madison
Schobert R, Jagusch C (2005) An expedient synthesis of 3-acyltetramic acids of the melophlin family from α-aminoesters and immobilized Ph3PCCO. Tetrahedron 61:2301–2307
Marquardt U, Schmid D, Jung G (2000) Racemic synthesis of the new antibiotic tetramic acid reutericyclin. Synlett 2000:1131–1132
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Acc Chem Res 41:157–167
Mikula H, Horkel E, Hans P, Hametner C, Fröhlich J (2013) Structure and tautomerism of tenuazonic acid—a synergetic computational and spectroscopic approach. J Hazard Mater 250–251:308–317
Acknowledgments
The Theodor Körner Fonds (Vienna, Austria) is gratefully acknowledged for financial support. We thank Philipp Hans for his support at the beginning of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary tables and figures, visualization of all optimized geometries, calculated energies for all reaction pathways considered, animations of all transition states, and Cartesian coordinates of all relevant structures. The electronic supplementary material (ESM) is available free of charge to subscribers at http://link.springer.de. (PDF 1798 kb)
ESM 2
(ZIP 34.6 mb)
Rights and permissions
About this article
Cite this article
Mikula, H., Svatunek, D., Skrinjar, P. et al. DFT study of the Lewis acid mediated synthesis of 3-acyltetramic acids. J Mol Model 20, 2181 (2014). https://doi.org/10.1007/s00894-014-2181-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2181-0