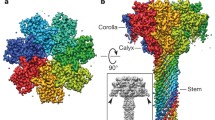
Abstract
During intoxication, the anthrax toxin lethal (LF) and edema (EF) factors initially assemble with the protective antigen (PA) on the plasma membrane of cells expressing the membrane-bound surface-exposed anthrax toxin receptor (ATR). This takes place at the physiological pH prior to entering the acidic environment of the endosome. We elucidated the molecular dynamics (MD) behaviors of the three-dimensional structure of the (PA63)7LF3 complex in various conformations and analyzed the dynamical properties of the fully loaded pre-pore complex on the plasma membrane at the physiological pH. The analysis points to the interaction networks of amino acids conserved between PA63 octamer and heptamer, which are not affected during the initial stage of the LFs binding. The simulations show an asymmetrical movement of the complex domains that directly affect LFs conformations. The conformational and structural alterations of the 2β2-2β3 loops of PA subunits are associated with pore formation. The early conformational changes of the loops appear as they peel off from the domain 2 toward domain 4 of each PA subunit. The LFs unfold in 1α1 segments of their N-terminal initiating the early stage of the pre-pore formation. The results indicate instable regions within the complex and provide important clues concerning the detail of fluctuating residues of the LF-PA interface regions at the early steps of toxins translocation.









Similar content being viewed by others
References
Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR et al. (1998) Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734–737
Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M et al. (1998) Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun 248:706–711
Leppla SH (1982) Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci 79:3162–3166
Abrami L, Lindsay M, Parton RG, Leppla SH, van der Goot FG (2004) Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J Cell Biol 166:645–651
Lacy DB, Wigelsworth DJ, Melnyk RA, Harrison SC, Collier RJ (2004) Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc Natl Acad Sci U S A 101:13147–13151
Santelli E, Bankston LA, Leppla SH, Liddington RC (2004) Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature 430:905–908
Thoren KL, Krantz BA (2011) The unfolding story of anthrax toxin translocation. Mol Microbiol 80:588–595
Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G (1992) Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem 267:16396–16402
Panchal RG, Halverson KM, Ribot W, Lane D, Kenny T et al. (2005) Purified bacillus anthracis lethal toxin complex formed in vitro and during infection exhibits functional and biological activity. J Biol Chem 280:10834–10839
Feld GK, Thoren KL, Kintzer AF, Sterling HJ, Tang II et al. (2010) Structural basis for the unfolding of anthrax lethal factor by protective antigen oligomers. Nat Struct Mol Biol 17:1383–1390
Elliott JL, Mogridge J, Collier RJ (2000) A quantitative study of the interactions of bacillus anthracis edema factor and lethal factor with activated protective antigen. Biochemistry 39:6706–6713
Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG (2003) Anthrax toxin triggers endocytosis of its receptor via a lipid raft–mediated clathrin-dependent process. J Cell Biol 160:321–328
Bann JG (2012) Anthrax toxin protective antigen—insights into molecular switching from prepore to pore. Protein Sci 21:1–12
Krantz BA, Trivedi AD, Cunningham K, Christensen KA, Collier RJ (2004) Acid-induced unfolding of the amino-terminal domains of the lethal and edema factors of anthrax toxin. J Mol Biol 344:739–756
Collier RJ, Young JAT (2003) Anthrax toxin. Annu Rev Cell Dev Biol 19:45–70
Scobie HM, Young JAT (2005) Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol 8:106–112
Jiang J, Pentelute BL, Collier RJ, Zhou ZH (2015) Atomic structure of anthrax protective antigen pore elucidates toxin translocation. Nature
Pannifer AD, Wong TY, Schwarzenbacher R, Renatus M, Petosa C et al. (2001) Crystal structure of the anthrax lethal factor. Nature 414:229–233
Scobie HM, Rainey GJA, Bradley KA, Young JAT (2003) Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci 100:5170–5174
Wynia-Smith SL, Brown MJ, Chirichella G, Kemalyan G, Krantz BA (2012) Electrostatic ratchet in the protective antigen channel promotes anthrax toxin translocation. J Biol Chem 287:43753–43764
Mogridge J, Cunningham K, Lacy DB, Mourez M, Collier RJ (2002) The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc Natl Acad Sci 99:7045–7048
Mogridge J, Cunningham K, Collier RJ (2001) Stoichiometry of anthrax toxin complexes. Biochemistry 41:1079–1082
Shen Y, Zhukovskaya NL, Guo Q, Florian J, Tang W-J (2005) Calcium-independent calmodulin binding and two-metal-ion catalytic mechanism of anthrax edema factor. EMBO J 24:929–941
Gogol EP, Akkaladevi N, Szerszen L, Mukherjee S, Chollet-Hinton L et al. (2013) Three dimensional structure of the anthrax toxin translocon–lethal factor complex by cryo-electron microscopy. Protein Sci 22:586–594
Ren G, Quispe J, Leppla SH, Mitra AK (2004) Large-scale structural changes accompany binding of lethal factor to anthrax protective antigen: a cryo-electron microscopic study. Structure 12:2059–2066
Chen R, Weng Z (2002) Docking unbound proteins using shape complementarity, desolvation, and electrostatics. Protein: Struct Funct Bioinform 47:281–294
Chen R, Weng Z (2003) A novel shape complementarity scoring function for protein-protein docking. Protein: Struct Funct Bioinform 51:397–408
Chaudhury S, Berrondo M, Weitzner BD, Muthu P, Bergman H et al. (2011) Benchmarking and analysis of protein docking performance in Rosetta v3.2. PLoS ONE 6, e22477
Wang C, Bradley P, Baker D (2007) Protein–protein docking with backbone flexibility. J Mol Biol 373:503–519
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE et al. (2005) GROMACS: fast, flexible, and free. J Comput Chem 26:1701–1718
Oostenbrink C, Villa A, Mark AE, Van Gunsteren WF (2004) A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J Comput Chem 25:1656–1676
Hess B (2008) P-LINCS: a parallel linear constraint solver for molecular simulation. J Chem Theory Comput 4:116–122
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Essmann U, Perera L, Berkowitz M, Darden T, Lee H et al. (1995) A smooth particle mesh Ewald method. J Chem Phys 103:17
Berendsen HJC, Postma JPM, Gunsteren WV, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 7
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:014101–014107
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Nosé S, Klein ML (1983) Constant pressure molecular dynamics for molecular systems. Mol Phys 50:1055–1076
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Shirts MR (2012) Simple quantitative tests to validate sampling from thermodynamic ensembles. J Chem Theory Comput 9:909–926
Gromacs (2010) The Gromacs user manual, version 4.5.6. http://www.gromacs.org/
Lacy DB, Lin HC, Melnyk RA, Schueler-Furman O, Reither L et al. (2005) A model of anthrax toxin lethal factor bound to protective antigen. Proc Natl Acad Sci U S A 102:16409–16414
Cunningham K, Lacy DB, Mogridge J, Collier RJ (2002) Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc Natl Acad Sci 99:7049–7053
Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC (1997) Crystal structure of the anthrax toxin protective antigen. Nature 385:833–838
Mourez M, Yan M, Lacy DB, Dillon L, Bentsen L et al. (2003) Mapping dominant-negative mutations of anthrax protective antigen by scanning mutagenesis. Proc Natl Acad Sci 100:13803–13808
Kintzer AF, Thoren KL, Sterling HJ, Dong KC, Feld GK et al. (2009) The protective antigen component of anthrax toxin forms functional octameric complexes. J Mol Biol 392:614–629
Young JAT, Collier RJ (2007) Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem 76:243–265
van der Goot G, Young JA (2009) Receptors of anthrax toxin and cell entry. Mol Aspects Med 30:406–412
Naik S, Brock S, Akkaladevi N, Tally J, McGinn-Straub W et al. (2013) Monitoring the kinetics of the pH driven transition of the anthrax toxin prepore to the pore by biolayer interferometry and surface plasmon resonance. Biochemistry
Miller CJ, Elliott JL, Collier RJ (1999) Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38:10432–10441
Nguyen TL (2004) Three-dimensional model of the pore form of anthrax protective antigen. Structure and biological implications. J Biomol Struct Dyn 22:253–265
Wang J, Vernier G, Fischer A, Collier RJ (2009) Functions of phenylalanine residues within the beta-barrel stem of the anthrax toxin pore. PLoS One 4, e6280
Benson EL, Huynh PD, Finkelstein A, Collier RJ (1998) Identification of residues lining the anthrax protective antigen channel. Biochemistry 37:3941–3948
Ascenzi P, Visca P, Ippolito G, Spallarossa A, Bolognesi M et al. (2002) Anthrax toxin: a tripartite lethal combination1. FEBS Lett 531:384–388
Zhang S, Finkelstein A, Collier RJ (2004) Evidence that translocation of anthrax toxin’s lethal factor is initiated by entry of its N terminus into the protective antigen channel. Proc Natl Acad Sci U S A 101:16756–16761
Lacy DB, Mourez M, Fouassier A, Collier RJ (2002) Mapping the anthrax protective antigen binding site on the lethal and edema factors. J Biol Chem 277:3006–3010
Acknowledgments
The authors thank Compute Canada for providing free access to High Performance Computer Clusters. LA thanks Memorial University for the startup grant. IR is grateful to the Canadian Foundation for Innovation (CFI 12824 to IR), the Canadian Institutes for Health Research (CIHR) for the operating grant (MOP 86693) and the new Investigator award.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Energy funnel resulted from protein-protein docking, representing the abundance of conformations. (GIF 11 kb)
Figure S2
Alteration of PA63 during 100 ns of MD simulations. RMSD change of A. Complex (PA63)7LF3, B. Variation of Solvent Accessible Surface (GIF 15 kb)
Figure S3
Variation of B-factor in three LF ligands. The lighter color represents the segments with higher B-factor value. The light green segments at domain 4 undergo the highest fluctuations. The “hammer-like” motion of the domain 1- domain 4 of LFs during 100 as the simulation progresses from time step “p” to “n” and to “n + m”. The X-ray structure is overlaid on the MD frames at the t ps (red) and tn ps (green) in ribbon and tn+m ps (gray) in cartoon representation. (GIF 154 kb)
Figure S4
A. The above view of the complex of LFs (surface) and PA63 (ribbons) obtained from the frame MD simulations, LF domains 1 (for clarity domains 2-4 are not shown) and PA domains 1’-4 (purple, navy, brown and green ribbons), B. Interacting amino acids from LF (yellow) and PA (gray). (GIF 210 kb)
Rights and permissions
About this article
Cite this article
Alisaraie, L., Rouiller, I. Molecular assembly of lethal factor enzyme and pre-pore heptameric protective antigen in early stage of translocation. J Mol Model 22, 7 (2016). https://doi.org/10.1007/s00894-015-2878-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2878-8