Abstract
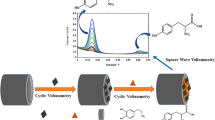
In this study, a novel modified glassy carbon electrode with copper polydopamine complex/multiwalled carbon nanotubes (GCE/Cu2+@PDA-MWCNTs) was fabricated and used for voltammetric determination of ascorbic acid (AA), dopamine (DA), acetaminophen (AC), nitrite (Nit), and xanthine (XN). Different techniques such as field emission electron microscopy, transmission electron microscopy, energy dispersive X-ray spectroscopy, Fourier-transform infrared spectroscopy, and electrochemical impedance spectroscopy were performed for characterization of the GCE/Cu2+@PDA-MWCNTs. Different electrochemical methods such as cyclic voltammetry, electrochemical impedance spectroscopy and differential pulse voltammetry (DPV) methods were employed to study the behavior of AA, DA, AC, Nit, and XN on this proposed modified electrode. The proposed modified electrode displays intense and indelible electrooxidation response for simultaneous determination of AA, DA, AC, Nit, and XN to five well-separated peaks in the potential range from 0.1 to 1.1 V using CV and DPV methods in phosphate buffer solution with pH 2.0. Under the optimum conditions, the calibration curves were liner up to 175, 125, 75, 150, and 115 μM with detection limits of 0.82, 0.45, 0.87, 0.92, and 0.67 μM for AA, DA, AC, Nit, and XN, respectively. This sensor was used to successfully determine these compounds in human urine and serum samples.

ᅟ





Similar content being viewed by others
References
Levine M (1986) New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med 314(14):892–902
Pauling L, Severinghaus JW (1976) Linus Pauling. National Public Radio
Wang G, Chen Z, Chen L (2011) Mesoporous silica-coated gold nanorods: towards sensitive colorimetric sensing of ascorbic acid via target-induced silver overcoating. Nanoscale 3(4):1756–1759
Khorasani-Motlagh M, Noroozifar M (2003) Electrocatalytic determination of L-ascorbic acid by modified glassy carbon with Ni (Me2 (CH3CO) 2 [14] tetraenoN4) complex. Anal Sci 19(12):1671–1674
Hallberg L (1981) Bioavailability of dietary iron in man. Annu Rev Nutr 1(1):123–147
Johnston CS (1999) Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 57(3):71–77
Matei N, Birghila S, Popescu V, Dobrinas S, Soceanu A, Oprea C, Magearu V (2008) Kinetic study of vitamin C degradation from pharmaceutical products. Rom J Phys 53(1–2):343–351
Pezzella A, d'Ischia M, Napolitano A, Misuraca G, Prota G (1997) Iron-mediated generation of the neurotoxin 6-hydroxydopamine quinone by reaction of fatty acid hydroperoxides with dopamine: a possible contributory mechanism for neuronal degeneration in Parkinson's disease. J Med Chem 40(14):2211–2216
Mo J-W, Ogorevc B (2001) Simultaneous measurement of dopamine and ascorbate at their physiological levels using voltammetric microprobe based on overoxidized poly (1, 2-phenylenediamine)-coated carbon fiber. Anal Chem 73(6):1196–1202
Pestana M, Jardim H, Serrao P, Soares-da-Silva P, Guerra L (1998) Reduced urinary excretion of dopamine and metabolites in chronic renal parenchymal disease. Kidney Blood Press Res 21(1):59–65
Graham GG, Scott KF, Day RO (2005) Tolerability of paracetamol. Drug Saf 28(3):227–240
Wangfuengkanagul N, Chailapakul O (2002) Electrochemical analysis of acetaminophen using a boron-doped diamond thin film electrode applied to flow injection system. J Pharm Biomed Anal 28(5):841–847
Filik H, Avan AA, Aydar S, Çetintaş G (2014) Determination of acetaminophen in the presence of ascorbic acid using a glassy carbon electrode modified with poly (caffeic acid). Int J Electrochem Sci 9:148–160
Huang Y-G, Ji J-D, Hou Q-N (1996) A study on carcinogenesis of endogenous nitrite and nitrosamine, and prevention of cancer. Mutat Res Fundam Mol Mech Mutagen 358(1):7–14
Yang YJ, Li W (2014) CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens Bioelectron 56:300–306
Amiri-Aref M, Raoof JB, Ojani R (2014) A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sensors Actuators B Chem 192:634–641
Sun L, Li H, Li M, Li C, Li P, Yang B (2016) Simultaneous determination of ascorbic acid, dopamine, uric acid, tryptophan, and nitrite on a novel carbon electrode. J Electroanal Chem 783:167–175
Cheemalapati S, Palanisamy S, Mani V, Chen S-M (2013) Simultaneous electrochemical determination of dopamine and paracetamol on multiwalled carbon nanotubes/graphene oxide nanocomposite-modified glassy carbon electrode. Talanta 117:297–304
Li F, Yang L, Zhao C, Du Z (2011) Electroactive gold nanoparticles/polyaniline/polydopamine hybrid composite in neutral solution as high-performance sensing platform. Anal Methods 3(7):1601–1606
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318(5849):426–430
Lee H, Rho J, Messersmith PB (2009) Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater 21(4):431–434
Lee H, Scherer NF, Messersmith PB (2006) Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci 103(35):12999–13003
Lee Y, Lee H, Kim YB, Kim J, Hyeon T, Park H, Messersmith PB, Park TG (2008) Bioinspired surface immobilization of hyaluronic acid on monodisperse magnetite nanocrystals for targeted cancer imaging. Adv Mater 20(21):4154–4157
Tao C, Yang S, Zhang J, Wang J (2009) Surface modification of diamond-like carbon films with protein via polydopamine inspired coatings. Appl Surf Sci 256(1):294–297
Kiss T, Gergely A (1979) Complexes of 3, 4-dhydroxyphnyl derivatives, III. Equilibrium study of parent and some mixed ligand complexes of dopamine, alanine and pyrocatechol with nickel (II), copper (II) and zinc (II) ions. Inorg Chim Acta 36:31–36
Huang N, Zhang S, Yang L, Liu M, Li H, Zhang Y, Yao S (2015) Multifunctional electrochemical platforms based on the Michael addition/Schiff base reaction of polydopamine modified reduced graphene oxide: construction and application. ACS Appl Mater Interfaces 7(32):17935–17946
Son HY, Ryu JH, Lee H, Nam YS (2013) Silver-polydopamine hybrid coatings of electrospun poly (vinyl alcohol) nanofibers. Macromol Mater Eng 298(5):547–554
Zhang Q-L, Xu T-Q, Wei J, Chen J-R, Wang A-J, Feng J-J (2013) Facile synthesis of uniform Pt nanoparticles on polydopamine-reduced graphene oxide and their electrochemical sensing. Electrochim Acta 112:127–132
Lin M, Huang H, Liu Y, Liang C, Fei S, Chen X, Ni C (2013) High loading of uniformly dispersed Pt nanoparticles on polydopamine coated carbon nanotubes and its application in simultaneous determination of dopamine and uric acid. Nanotechnology 24(6):065501
Yang L, Kong J, Zhou D, Ang JM, Phua SL, Yee WA, Liu H, Huang Y, Lu X (2014) Transition-metal-ion-mediated polymerization of dopamine: mussel-inspired approach for the facile synthesis of robust transition-metal nanoparticle–graphene hybrids. Chem Eur J 20(25):7776–7783
Paris I, Dagnino-Subiabre A, Marcelain K, Bennett LB, Caviedes P, Caviedes R, Azar CO, Segura-Aguilar J (2001) Copper neurotoxicity is dependent on dopamine-mediated copper uptake and one-electron reduction of aminochrome in a rat substantia nigra neuronal cell line. J Neurochem 77(2):519–529
Postma A, Yan Y, Wang Y, Zelikin AN, Tjipto E, Caruso F (2009) Self-polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chem Mater 21(14):3042–3044
Li H, Jia Y, Feng X, Li J (2017) Facile fabrication of robust polydopamine microcapsules for insulin delivery. J Colloid Interface Sci 487:12–19
Zangmeister RA, Morris TA, Tarlov MJ (2013) Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 29(27):8619–8628
Nakamoto K (1986) Infrared and raman spectra of inorganic and coordination compounds. Wiley Online Library
Ho C-C, Ding S-J (2014) Structure, properties and applications of mussel-inspired polydopamine. J Biomed Nanotechnol 10(10):3063–3084
Goss CA, Abruna HD (1985) Spectral, electrochemical and electrocatalytic properties of 1, 10-phenanthroline-5, 6-dione complexes of transition metals. Inorg Chem 24(25):4263–4267
Shahbakhsh M, Noroozifar M (2018) Poly (dopamine quinone-chromium (III) complex) microspheres as new modifier for simultaneous determination of phenolic compounds. Biosens Bioelectron 102:439–448
DuVall SH, McCreery RL (1999) Control of catechol and hydroquinone electron-transfer kinetics on native and modified glassy carbon electrodes. Anal Chem 71(20):4594–4602
Chen Z, Zhang Z, Qu C, Pan D, Chen L (2012) Highly sensitive label-free colorimetric sensing of nitrite based on etching of gold nanorods. Analyst 137(22):5197–5200
Wang C, Yuan R, Chai Y, Zhang Y, Hu F, Zhang M (2011) Au-nanoclusters incorporated 3-amino-5-mercapto-1, 2, 4-triazole film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens Bioelectron 30(1):315–319
Shahbakhsh M, Narouie S, Hashemzaei Z, Nouri A, Saravani H, Noroozifar M (2017) Modified graphite paste electrode with strontium phen-dione complex for simultaneous determination of a ternary mixture of dopamine, acetaminophen and xanthine (Part II). Int J Electrochem Sci 12:11763–11777
Zhang Y, Yuan R, Chai Y, Li W, Zhong X, Zhong H (2011) Simultaneous voltammetric determination for DA, AA and NO2− based on graphene/poly-cyclodextrin/MWCNTs nanocomposite platform. Biosens Bioelectron 26(9):3977–3980
Zhou Y, He M, Huang C, Dong S, Zheng J (2012) A novel and simple biosensor based on poly (indoleacetic acid) film and its application for simultaneous electrochemical determination of dopamine and epinephrine in the presence of ascorbic acid. J Solid State Electrochem 16(6):2203–2210
Ghica ME, Wintersteller Y, Brett CM (2013) Poly (brilliant green)/carbon nanotube-modified carbon film electrodes and application as sensors. J Solid State Electrochem 17(6):1571–1580
Li NB, Ren W, Luo HQ (2008) Simultaneous voltammetric measurement of ascorbic acid and dopamine on poly (caffeic acid)-modified glassy carbon electrode. J Solid State Electrochem 12(6):693–699
Vidyadharan AK, Jayan D, Nancy TM (2014) Ni 0.1 Co 0.9 Fe 2 O 4-based electrochemical sensor for the detection of paracetamol. J Solid State Electrochem 18(9):2513–2519
Yang C, Xu J, Hu S (2007) Development of a novel nitrite amperometric sensor based on poly (toluidine blue) film electrode. J Solid State Electrochem 11(4):514–520
Yang S, Li G, Yang R, Xia M, Qu L (2011) Simultaneous voltammetric detection of dopamine and uric acid in the presence of high concentration of ascorbic acid using multi-walled carbon nanotubes with methylene blue composite film-modified electrode. J Solid State Electrochem 15(9):1909–1918
Yang H, Li Y, Liu Y, Zhang Y, Zhao Y, Zhao M (2015) One-pot chemical blasting synthesis of the bamboo-like multiwalled carbon nanotubes/graphene oxide nanocomposite and its application in electrochemical detection of dopamine. J Solid State Electrochem 19(1):145–152
Zhang L, Shi Z, Lang Q (2011) Fabrication of poly (orthanilic acid)–multiwalled carbon nanotubes composite film-modified glassy carbon electrode and its use for the simultaneous determination of uric acid and dopamine in the presence of ascorbic acid. J Solid State Electrochem 15(4):801–809
Zhou S, Zuo R, Zhu Z, Wu D, Vasa K, Deng Y, Zuo Y (2013) An eco-friendly hydrophilic interaction HPLC method for the determination of renal function biomarkers, creatinine and uric acid, in human fluids. Anal Methods 5(5):1307–1311
Rincón F, Martínez B, Delgado J (2003) Detection of factors influencing nitrite determination in meat. Meat Sci 65(4):1421–1427
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shahbakhsh, M., Noroozifar, M. Copper polydopamine complex/multiwalled carbon nanotubes as novel modifier for simultaneous electrochemical determination of ascorbic acid, dopamine, acetaminophen, nitrite and xanthine. J Solid State Electrochem 22, 3049–3057 (2018). https://doi.org/10.1007/s10008-018-4013-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-4013-0