Abstract
A critical goal of HIV vaccine development is the identification of safe and immunogenic vectors. Recombinant vaccinia virus is a highly effective vaccine vector, with demonstrated capacity to protect animals from various viral pathogens, including rabies. Unlike many other candidate vaccine vectors, vast human experience exists with the parenteral smallpox vaccine. However, consideration of recombinant vaccinia virus as a modern vaccine is complicated by the relatively high prevalence of immunocompromised persons compared to such prevalence 4 or more decades ago (when smallpox vaccination was still routine). Administering vaccine by the subcutaneous (SQ) route, rather than the traditional scarification route, could address these concerns. SQ administration could prevent transmission of vaccinia virus to potentially vulnerable persons; it could also avoid the most common adverse events, which are cutaneous in nature. However, previous studies suggest that elicitation of immune response against passenger gene products following SQ administration requires development of a superficial pox lesion, defeating the intention of SQ administration. This is the first report to demonstrate that SQ administration of recombinant vaccinia virus does elicit immune response to the passenger protein in the absence of a cutaneous pox lesion. Results further show that a multi-envelope HIV vaccine can elicit antibody responses toward heterologous HIV-1 not represented by primary sequence in the vaccine. These findings have global implications because they support the consideration of recombinant vaccinia virus as a valuable HIV vaccine vector system.
Similar content being viewed by others
Introduction
An effective HIV vaccine must overcome two substantial obstacles: the identification of safe and immunogenic vaccine vectors; and the antigenic diversity of HIV. To address these challenges, we have developed a prime-boost-boost vaccine strategy including recombinant DNA, vaccinia virus and purified protein, designed to encompass the antigenic diversity of HIV. As a first step in testing this three-tiered approach in humans, we prepared and evaluated PolyEnv1, a recombinant vaccinia virus vaccine formulated with 23 recombinants, each expressing a distinct HIV-1 envelope [1, 2]. PolyEnv1 is the first such vaccine to enter clinical trial. Vaccinia virus (VV), naturally attenuated in humans, undergoes limited replication following inoculation and effectively stimulates long-lasting B- and T-cell responses [3], evident in the success of the global smallpox eradication campaign.
Unlike any other live-virus vector candidates, VV has been administered to millions of humans, providing a comprehensive registry of vaccine-related adverse events [4]. When administered by superficial scarification, the vaccine may be highly transmissible and can rarely cause cutaneous complications (e.g. generalized vaccinia, eczema vaccinatum, progressive vaccinia) in vulnerable populations. Since HIV vaccines will ultimately be administered to HIV-negative persons living in HIV-positive communities, possible transmission of vaccine virus is a concern.
To avoid potential inadvertent transmission or cutaneous complications of vaccination, we examined the SQ route of inoculation of a novel recombinant VV vaccine. Previous studies of HIV-1 envelope recombinant VV vaccines administered SQ suggested that envelope-specific immunity required development of a cutaneous pox lesion [5], eliminating the advantages of the SQ route. To further investigate this, we prepared recombinant VV constructs expressing HIV envelope (gp140) and administered this vaccine by the SQ route.
Here we report the elicitation of human immune responses to envelope-recombinant VV in the absence of a cutaneous pox lesion. This is the first report to demonstrate the value of this approach and to suggest a valuable and safe route for recombinant VV that should be explored in larger studies.
Materials and Methods
Vaccine Design
To address the diversity of HIV, we have developed a prime-boost-boost vaccination strategy consisting of DNA, vaccinia virus and protein recombinants [1, 2] designed to encompass the following: (i) envelope quasispecies from longitudinal sampling of infected individuals [6]; (ii) envelopes with diverse patterns of monoclonal antibody binding [7]; and (iii) envelopes representing multiple clades. Vaccine envelope sequences were PCR-amplified directly from the blood of infected persons or from virus passaged briefly in vitro on peripheral blood mononuclear cells. Twenty-three distinct vaccinia virus HIV-1-envelope constructs (designated VVenv), each expressing a distinct envelope protein, were prepared using previously described methods [8]. Each individual VVenv expressed the gp120 protein and the extracellular portion of gp41 (as gp140 oligomers). The 23 VVenv constructs were expanded individually and combined into a multi-envelope vaccine (designated PolyEnv1) in a dedicated facility at St. Jude Children’s Research Hospital.
Study Design
A study evaluating the tolerability and safety of SQ PolyEnv1 was reviewed and approved by the Food and Drug Administration and the St. Jude Children’s Research Hospital Institutional Review Board. Inclusion criteria required subjects to be healthy HIV-negative adults over 18 years of age without previous smallpox vaccination. Volunteers were excluded if they were at high-risk for HIV or had immunosuppressed household contacts. Informed consent was given by all subjects.
For vaccination, 105 pfu of PolyEnv1 was administered SQ over the posterior triceps muscle using a tuberculin syringe. To avoid the development of ulcerative pox lesions, we followed three practices aimed at reducing potential contamination of skin surfaces with vaccine. First, the needle was changed after vaccine was drawn into the syringe to exclude virus from the needle exterior. Second, vaccine was administered using SQ “z-tracking”, whereby skin was pulled laterally before needle insertion and released before needle withdrawal to create an indirect or “z” path to reduce vaccine seepage to skin surface. Third, the needle was retained in place for 10 s following injection to prevent vaccine egress along the needle track. Injection sites were wiped with alcohol after injection and covered with a transparent impermeable dressing (OpSite; Smith & Nephew, Largo, FL, USA) that was removed only during clinic visits over the first 28 days. At the time of this report, all three recipients had been followed for 52 weeks.
Laboratory Assays
Blood was obtained at baseline and periodic clinic visits for complete blood counts with differential, platelet count, T- and B-cell subsets, serum alanine aminotransferase, aspartate aminotransferase, creatinine, and HIV-1 RNA (Nuclisens NASBA kit; Organon Teknika, Durham, NC, USA). Antibody was measured by commercial HIV enzyme-linked immunosorbent assay (ELISA) (Abbott laboratories, Abbott Park, IL, USA) and Western blot assays (Organon Teknika). The Abbott ELISA is routinely used in hospitals and clinics for the diagnosis of HIV infection. The false-positive rate is low and recipients of the smallpox vaccine (most Americans born before the year 1971 and researchers working with vaccinia virus in the laboratory) generally score negative in this assay, unless they are infected with HIV.
A VV-specific ELISA (1:10 serum dilution) was performed using plates coated with purified wild-type VV (Western Reserve vaccinia virus, 1:3000 dilution) derived from lysates of infected fibroblasts. The assay was developed with anti-human Ig conjugated to alkaline phosphatase, followed by p-nitrophenyl phosphate.
Neutralization Assay
To detect functional antibody directed against the recombinant gene products expressed by PolyEnv1, sera (serial 2-fold dilutions from 1:20 to 1:80) were tested in replicate in a blinded fashion on two occasions for neutralizing activity. Virus 320f-98 was a clinical isolate (heterologous by sequence analysis to the 23 envelope sequences included in PolyEnv1; data not shown) selected for neutralization studies and passaged fewer than three times in peripheral blood mononuclear cells before short-term growth on MT2 cells.
Standard MT-2-based neutralization assays were performed. Briefly, sera (20 µl) were pre-incubated with virus (20 µl; 5–25 TCID50) for 60 min at room temperature in a 96-well microtiter plate, after which MT-2 cells were added (104/well). After a 7-day incubation period (at 37°C/5% CO2), syncytia were counted or samples were tested for HIV-1 p24 antigen by ELISA (Coulter; Westbrook, ME, USA). Normal human serum samples (from unvaccinated individuals) were tested as negative controls, and none scored positive. Sera from vaccinees were compared pre- and post-vaccination for the development of neutralizing responses.
Results
Subjects
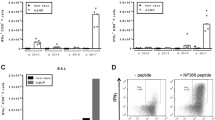
Two males and one female were vaccinated with SQ PolyEnv1. One vaccinee (001) experienced mild pruritus which began 6 h after injection and resolved spontaneously 6 h thereafter. Two of the three subjects (001, 002) developed moderate swelling, erythema (7 cm×6 cm and 10 cm×11 cm, respectively; Fig. 1) and induration at the vaccine site with ipsilateral axillary adenopathy. Reactions were first noted 14 and 10 days following vaccination for vaccinees 001 and 002, respectively. Local signs of SQ virus replication resolved spontaneously within 2–3 days and ulcerative pox lesions never developed (Fig. 1). One vaccinee (002) had low-grade fever and malaise accompanying local symptoms. Vaccinee 003 did not manifest any local signs or symptoms following vaccination.
Sample local response to subcutaneous vaccination. Ten days after inoculation vaccinee 002 developed erythema and induration at the site of subcutaneous vaccination (second panel). Local signs resolved within 3 days (third panel) and complete healing without development of a cutaneous pox lesion was evident at 28 days (last panel)
Clinical Laboratory Results
No laboratory abnormalities were noted apart from a transient reversal of the CD4:CD8 ratio (from 2.3 to 0.3) 2 weeks following vaccination of one individual (003), with spontaneous resolution noted 4 weeks later. HIV PCR testing remained negative for all subjects throughout the study.
Antibody Responses
To evaluate the capacity for SQ-administered recombinant VV to elicit immune responses, we measured sera for antibody responses against both VV and the recombinant antigen (HIV-1 envelope). Studies demonstrated that the two subjects who developed local vaccine reactions (001, 002) also developed VV-specific antibody responses, first detected between 4 and 8 weeks after vaccination (Fig. 2A). VV-specific antibody responses peaked at different times after vaccination for each of the 2 volunteers (4 months for 001 and 10 weeks for 002). VV-specific antibody responses were not detected in the vaccinee (003) who failed to develop a local vaccine reaction.
Subcutaneous PolyEnv1 vaccination elicits vaccinia virus-specific and HIV-envelope-specific antibody. Serum samples collected at the specified times post-immunization for each vaccinee (x-axis) were analyzed for A vaccinia virus-specific or B envelope-specific antibody responses, each by ELISA. A commercial ELISA assay (Abbott) was used to measure HIV responses. This kit measures antibodies against the HIV IIIB envelope. Antibody levels are reported as OD units measured at 405 nm (y-axis). The assay cut-off for the Abbott HIV ELISA is 0.11 units. C In addition to measuring ELISA responses, sera from vaccinee 002 obtained pre- and post-vaccination were examined for neutralization of heterologous HIV-1 320f-98. Sera were examined in duplicate at 1:20, 1:40 and 1:80 dilutions at each time point. Neutralizing activity against virus grown on MT-2 cells was measured by p24 content and by syncytia formation, performed after 7 days of incubation. Results show evidence of virus neutralization at 1:20 and 1:40 dilutions (in bold) of post-vaccination but not pre-vaccination serum
We next examined vaccinee sera for HIV-1-specific antibodies using a commercial ELISA (Abbott) based on HIV-1 IIIB envelope antigens. The IIIB HIV-1 envelope in the ELISA did not share genetic sequence with any of the 23 envelopes in PolyEnv1. This ELISA detected envelope-specific antibody responses from both of the volunteers who manifested a local vaccine reaction and VV-specific antibodies (Fig. 2B). Responses against the IIIB envelope were first detected between 8 and 10 weeks following vaccination for both vaccinees. As with the VV-specific responses, the IIIB-specific antibody responses peaked at different times for the two vaccinees (4 months for vaccinee 001 and 10 months for vaccinee 002), reaching levels more than 10 times that of the pre-vaccination controls. Elevated ELISA responses against IIIB envelope were still detectable in vaccinee 002 at the last study point (12 months). HIV-1 Western blot testing remained negative throughout the study. As expected, HIV-1-specific responses were not observed from the vaccinee (003) who failed to develop a local response to vaccination or anti-VV antibody responses.
We next tested those sera that scored positive in HIV-1 envelope ELISA for the capacity to neutralize HIV-1 in vitro. Serum samples from vaccinees 001 and 002 (obtained 4 months post-vaccination) were evaluated for neutralizing activity against a clinical isolate of HIV-1 (320f-98). The envelope gene sequence of isolate 320f-98 was distinct from all the envelope sequences included in PolyEnv1 (data not shown). Whereas neutralization was not evident with pre-immunization serum, post-immunization serum from vaccinee 002 demonstrated neutralizing activity against heterologous 320f-98 at serum titers of 1:20 and 1:40 (Fig. 2C). These reproducible results were measured by both reduction of p24 production and lack of syncytia formation. Evidence of neutralizing activity at 1:40 serum dilution at 4 months post-vaccination was also identified from vaccinee 001, but only in one of two assays (data not shown).
Discussion
In the study reported here we investigated the consequences of SQ administration of a novel recombinant VV vaccine. We employed the SQ route to avoid potential cutaneous dissemination or potential transmission of virus between individuals. In contrast to previous proposals [5, 9, 10], we hypothesized that careful SQ administration would allow replication of the vaccine vector and the elicitation of antibody responses to the passenger gene product without ulcer formation. Our hypothesis proved correct: two of three subjects receiving 105 pfu of SQ PolyEnv1 developed signs of VV replication (i.e., erythema, induration and ipsilateral adenopathy) without superficial pox lesion development. The two vaccinees with evidence of SQ virus replication both developed antibody responses to the vector and to the HIV-1 envelope. It is possible that inadvertent intramuscular inoculation of the third vaccinee (003) may have precluded virus replication altogether, explaining the lack of local signs and of antibody responses [9].
The capacity for SQ VV to elicit immune responses was first demonstrated over 65 years ago [11]: recipients of SQ smallpox vaccine were protected from percutaneous re-challenge with smallpox vaccine, demonstrating that primary SQ inoculation had primed effective immunity. While vaccination resulting in ulcerative pox lesions may yield more potent responses [12], here we show that specific immunity can be elicited in the absence of transmissible lesions. Indeed, our results contrast with those of a previous study of an alternate, single envelope-recombinant VV vaccine, for which it was reported that antibody responses were not elicited unless a cutaneous lesion was evident [5]. We anticipate that the modest responses elicited here with VV alone will be amplified following administration of our full prime-boost-boost, multi-envelope regimen of DNA, VV and purified protein [2].
In addition to examining the utility of recombinant VV administered by the SQ route, we tested the capacity for a multi-envelope HIV vaccine to elicit a breadth of envelope-specific immune responses. We have hypothesized that delivery of a cocktail of antigenically distinct HIV envelopes should activate immune responses capable of recognizing natural isolates of HIV [1, 2, 13]. This study serves as a proof of principle supporting this hypothesis. The administration of PolyEnv1-stimulated antibody responses capable of recognizing HIV isolates not represented by primary sequence in the vaccine (i.e. IIIB and 320f-98). While not represented by primary sequence, it is evident that antigenic determinants are present among the 23 envelopes included in PolyEnv1, which are shared by isolates that differ in primary amino acid sequence. This occurs because different linear amino-acid sequences may fold to create similar conformational B-cell epitopes. Indeed, most HIV envelope B-cell determinants are conformational [14], and there may be a great redundancy in envelope conformation due to constraints dictated by functional requirements (e.g. binding cell surface receptors). This redundancy would predict that multi-envelope formulations representing the diverse HIV-1 envelope structures in nature can be feasibly prepared.
This study thus demonstrates the immunogenicity of recombinant VV vector delivered by the SQ route. Preliminary data further suggest that this vector can be used to administer multiple envelopes to trigger broad responses, and it can do so in the absence of a transmissible pox lesion. Given the historical successes of VV, and the option to deliver this vector by the SQ route, VV may prove to be a promising HIV vaccine delivery system.
References
Lockey TD, Slobod KS, Caver TE, D’Costa S, Owens RJ, McClure HM, Compans RW, Hurwitz JL (2000) Multi-envelope HIV vaccine safety and immunogenicity in small animals and chimpanzees. Immunol Res 21:7–21
Caver TE, Lockey TD, Srinivas RV, Webster RG, Hurwitz JL (1999) A novel vaccine regimen utilizing DNA, vaccinia virus and protein immunizations for HIV-1 envelope presentation. Vaccine 17:1567–1572
Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK (2003) Duration of antiviral immunity after smallpox vaccination. Nat Med 9:1131–1137
Centers for Disease Control and Prevention (2003) Smallpox vaccination and adverse reactions: guidance for clinicians. MMWR Dispatch 52:1–29
Graham BS, Belshe RB, Clements ML, et al (1992) Vaccination of vaccinia-naïve adults with human immunodeficiency virus type 1 gp160 recombinant vaccinia virus in a blinded, controlled, randomized clinical trial. J Infect Dis 166:244–252
Rencher SD, Slobod KS, Dawson D, Lockey TD, Hurwitz JL (1995) Does the key to a successful HIV vaccine lie among the envelope sequences of infected individuals? AIDS Res Hum Retroviruses 11:1131–1133
Rencher SD, Hurwitz JL (1997) Effect of natural HIV-1 envelope V1-V2 sequence diversity on the binding of V3 and non-V3-specific antibodies. J Acquir Immune Defic Syndr 16:69–73
Ryan KW, Owens RJ, Hurwitz JL (1997) Preparation and use of vaccinia virus vectors for HIV protein expression and immunization. In: Lefkovits I (ed) Immunology methods manual. Academic Press, New York, pp 1993–2015
Cieslak TJ, Christopher GW, Kortepeter MG, et al (2000) Immunization against potential biological warfare agents. Clin Infect Dis 30:843–850
McClain DJ, Harrison S, Yeager CL, et al (1997) Immunologic responses to vaccinia vaccines administered by different parenteral routes. J Infect Dis 175:756–763
Connors JD, McIntosh K, Cherry JD, et al (1977) Primary subcutaneous vaccination. J Infect Dis 135:167–175
Cooney EL, Collier AC, Greenberg PD, et al (1991) Safety of immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet 337:567–572
Slobod KS, Rencher SD, Farmer A, Smith FS, Hurwitz JL (1994) HIV type 1 envelope sequence diversity in an inner city community. AIDS Res Hum Retroviruses 10:873–875
Moore JP, Ho DD (1993) Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol 67:863–875
Acknowledgements
Sources of financial support: P01 AI45142 (NIAID, NIH for KSS, PCD and JLH). This work was supported in part by Cancer Center Support Grant No P30-CA21765 (NCI) and the American Lebanese Syrian Associated Charities (ALSAC). TDL was supported by a national Research Service Award 5T32-CA09346. We thank B. Williams, M. Roy, J. Parobek and J. Zacher for their assistance; W.T. Hughes and J.W. Sixbey, P.M. Flynn for helpful discussion. We thank H. Stamey and the Tennessee Blood Service (Memphis, TN). We thank the NIH AIDS Research and Reference Reagent Repository and WHO/UNAIDS for providing envelope genes representative of different clades. We are grateful to the volunteers without whom the study would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slobod, K.S., Lockey, T.D., Howlett, N. et al. Subcutaneous Administration of a Recombinant Vaccinia Virus Vaccine Expressing Multiple Envelopes of HIV-1. Eur J Clin Microbiol Infect Dis 23, 106–110 (2004). https://doi.org/10.1007/s10096-003-1075-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-003-1075-3