Abstract
The purpose here is to establish the incidence of respiratory tract colonization with Candida (RT Candida) among ICU patients receiving mechanical ventilation within studies in the literature. Also of interest is its relationship with candidemia and the relative importance of topical antibiotic (TA) use as within studies of selective digestive decontamination (SDD) versus other candidate risk factors towards it. The incidence of RT Candida was extracted from component (control and intervention) groups decanted from studies of various TA and non-TA ICU infection prevention methods with summary estimates derived using random effects. A benchmark RT Candida incidence to provide overarching calibration was derived using (observational) groups from studies without any prevention method under study. A multi-level regression model of group level data was undertaken using generalized estimating equation (GEE) methods. RT Candida data were sourced from 113 studies. The benchmark RT Candida incidence is 1.3; 0.9–1.8 % (mean and 95 % confidence intervals). Membership of a concurrent control group of a study of SDD (p = 0.02), the group-wide presence of candidemia risk factors (p < 0.001), and proportion of trauma admissions (p = 0.004), but neither the year of study publication, nor membership of any other component group, nor the mode of respiratory sampling are predictive of the RT Candida incidence. RT Candida and candidemia incidences are correlated. RT Candida incidence can serve as a basis for benchmarking. Several relationships have been identified. The increased incidence among concurrent control groups of SDD studies cannot be appreciated in any single study examined in isolation.
Similar content being viewed by others
Introduction
Respiratory tract colonization with Candida (RT Candida) among patients with suspected ventilator-associated pneumonia (VAP) has been reported in numerous studies [1–113]. Both the overall incidence and the clinical significance for the individual patient are uncertain. True Candida pneumonia in this patient group is thought to be rare [114, 115]. Among a tally of 2,490 isolates from 24 studies, fungi (species unspecified) accounted for only 0.9 % of pathogenic isolates [116]. While current guidelines [114, 115] do not recommend treatment of RT Candida, it remains of interest for at least four reasons.
Firstly, colonization with Candida is believed to be a key intermediary step towards invasive candidiasis, although the role of RT Candida in this respect is unclear. The respiratory tract, being a site not normally colonized by Candida, may provide a unique insight into factors influencing the incidence of Candida colonization. Thirdly, RT Candida may be a risk factor for specific bacterial infections due to molecular interactions [117, 118].
Finally, the influence of topical antibiotic (TA) use as a method to prevent ICU-acquired bacterial colonization and infection, as within studies of selective digestive decontamination (SDD) and selective oro-pharyngeal decontamination (SOD) [119, 120], on the incidence of Candida colonization is of longstanding interest. This question was raised in the first study of SDD [74]. There appear to be subtle contextual effects of using topical antibiotics in the ICU on the incidence of candidemia [121], as with bacteremia [122] and also as with ventilator-associated pneumonia [123], which are evident only through benchmarking the control group rates of these studies and which are not seen in studies of non-TA methods of ICU infection prevention.
RT Candida data is available from numerous studies of a broad range of VAP prevention methods which have been reviewed in systematic reviews [119, 120, 124–135]. Among this evidence base are those with concurrent versus non-concurrent study designs, together with other study designs including those without any intervention. This heterogeneous evidence base provides a natural experiment [136, 137] with which to address some of these questions at the group level, using methods as used in the analysis of cluster randomized trials.
Materials and methods
Study selection and decant of groups
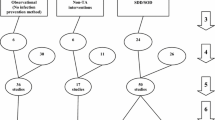
The literature search and analytic approach used here is as described previously [121]. These seven steps (Fig. 1; numbered arrows) are as follows;
Search method, screening criteria, and resulting classification of eligible studies and subsequent decant of component groups. The seven numbered arrows to the right represent steps in the process as discussed in the methods; steps 1 to 5 refer to studies, and steps 6 and 7 refer to component groups decanted from the studies being control (rectangles) and intervention (ovals) groups from ICU-based studies of infection prevention methods and observation groups from cohorts of ICU patients without a prevention method under study (diamond). The horizontal dotted rectangles represent the group contrasts used toward the calculation of the contextual effects of the component groups, each versus the observation groups as the reference category. Note; the total numbers do not tally, as some systematic reviews provided studies in more than one category, and some studies provided groups in more than one category. Note; SDD includes SOD and other methods based on the use of a topical antibiotic (TA)
-
1.
An electronic search of PubMed, The Cochrane database and Google Scholar for systematic reviews containing potentially eligible studies was undertaken using the following search terms; “ventilator-associated pneumonia”, “mechanical ventilation”, “intensive care unit”, each combined with either “meta-analysis” or “systematic review” up to December 2013.
-
2.
Systematic reviews of studies of patient populations requiring prolonged (>24 hours) ICU admission were then streamed into one of three categories; systematic reviews containing studies in which there was no intervention, studies with SDD as the intervention, or studies with an intervention other than SDD, for the prevention of VAP. For the purpose of this study, SDD is defined here as the use of protocolized topical antibiotic prophylaxis applied by the gastric or oro-pharyngeal route in the intervention group, with or without the additional use of a parenteral antibiotic or any anti-fungal agent.
-
3.
The studies were screened against the following eligibility criteria. Inclusion criteria; incidence data for ventilator-associated pneumonia extractable as an incidence proportion being expressed as a proportion of numbers of patients among patients with an ICU stay of at least 24 hours. Exclusion criterion; studies limited to patients with the acute respiratory distress syndrome. Studies in a language other than English were included when the required data had been abstracted in an English-language systematic review.
-
4.
A hand search was undertaken for additional studies not identified within systematic reviews but otherwise meeting the eligibility criteria.
-
5.
All eligible studies were then collated and any duplicate studies were removed.
-
6.
Groups of patients receiving mechanical ventilation from studies without a VAP prevention method under study were labelled as observational groups. The studies of intervention studies were classified as follows. The non-TA-based methods of VAP prevention used interventions other than topical antibiotics. These were usually delivered at either the gastric, airway, or oral sites. The SDD studies were further sub-classified as to whether the control group was concurrent and co-located within the same ICU as the intervention group (concurrent control) or not (non-concurrent).
-
7.
The component (control and intervention) groups were decanted from each study as follows;
-
The control and intervention groups from non-TA based methods were classified as indicated in the original study
-
Among studies of SDD, all groups that received prophylaxis with any regimen of topical antibiotic, whether or not an anti-fungal was included in the regimen, were designated as an SDD intervention group and all other groups from SDD studies were classified as a control group.
-
Data extraction
The RT Candida figure is the number of patients with Candida isolates from respiratory sampling per 100 patients with prolonged (>24 hours) stay in the ICU, whether or not VAP has been documented. In addition, the following were also extracted where available; the overall incidence proportion of VAP, the incidence of candidemia, the incidence of Candida colonization at non-respiratory tract sites without regard to how this had been defined in each study, and the proportion of admissions for trauma. Each of these were expressed as a proportion, using the number of patients with prolonged (>24 hours) stay in the ICU as the denominator. Other parameters extracted were whether the mode of diagnosis of VAP required bronchoscopic sampling and whether topical placebo had been used to achieve observer blinding.
Caterpillar plots
To generate caterpillar plots, the RT Candida data were logit transformed for analysis as previously [137]; with the total number of patients as the denominator (D), the number of patients with RT Candida as the numerator (N), and R being the RT Candida proportion (N/D), the logit(RT Candida) is log(N/(D−N)) and its variance is 1/(D*R*(1–R)). Note that for any group with a zero event rate (N = 0), the addition of the continuity correction (i.e., N + 0.5) is required to avoid indeterminate transformations of mean and variance. Using these pre-calculated logits and logit variances, group specific 95 % confidence intervals, summary logits and the associated summary 95 % CIs were generated using the ‘metan’ command in STATA. On the logit scale, the 95 % confidence intervals for a proportion are symmetrical and remain within the interval of 0 to 100 %.
For each category of component group the summary mean logit RT Candida and associated 95 % confidence interval were calculated using random effects methods. These were then back-transformed to the percentage scale. The benchmark is the summary mean RT Candida per 100 patients derived from the observational studies, and the benchmark range is the 95 % prediction interval.
Bivariate plots and confidence ellipses
To assess correlation of RT Candida with candidemia incidence, the logit-transformed data was assessed by two methods; a 95 % prediction ellipse [138–140], and linear regression. The prediction ellipse method enables the correlation as observed in other studies to be benchmarked. The relationship between logit-transformed RT Candida with year of publication was assessed using locally weighted regression and smoothing scatterplot (LOWESS) [141].
Statistical analysis
A regression model of RT Candida proportion was developed using GEE methods, as these accommodate any intra-cluster correlation (‘xtgee’ command in STATA; release 12.0, STATA Corp., College Station, TX, USA). In this analysis, the predictor variables were: (1) the component group membership, being either membership of a group from an observational study, a control group, or an intervention group, (2) type of intervention under study, (3) the use or non-use of topical placebo, and (4) whether the mode of diagnosis of VAP required bronchoscopic sampling. As a sensitivity analysis, the GEE regression model analysis was repeated limited to studies obtained from systematic reviews. In addition to the Poisson model, the GEE regression model was undertaken with both binomial and negative binomial models as additional sensitivity tests.
Results
Characteristics of the studies
Of the 113 studies identified by the search [1–113], 72 were sourced from 13 systematic reviews and a further 40 sourced from elsewhere (Table 1; Fig. 1). The majority of SDD studies were published in the 1990s, and all but four studies of SDD were European in origin. Two studies were supplemented with data from published doctoral theses [76, 113] or related publications [74].The studies are detailed in the Electronic Supplementary Material (ESM) file.
A total of 191 component groups were decanted from these 113 studies, with 36 groups from observational studies (ESM file Table S1), 71 groups from studies of various non-TA methods of VAP prevention (ESM file Table S2), and 84 groups from studies of SDD having either a non-concurrent (ESM file Table S3) or concurrent design (ESM file Table S4). Eleven studies had more than one observational, control, or intervention group. Two studies had both concurrent and non-concurrent control groups. Group-wide risk factors for candidemia were identified in only six studies. Three SDD studies used a regimen not containing an anti-fungal [71, 111, 112].
Candida colonization
A measure of Candida colonization not limited to patients with suspected VAP and not limited to respiratory sites was reported for 32 studies including 18 of the SDD studies (Table 1). There was a wide range in this incidence (Figs. S5 & S6), with the incidence among concurrent control (p = 0.066; Table S5) groups from studies of SDD being higher versus the incidence in the observational groups.
The incidence of Candida colonisation, at sites other than the respiratory tract and not restricted to patients with suspected VAP, together with candidemia incidence, were available from 40 groups from studies of all types. There were too few groups to discern a significant relationship between these incidences or to generate a robust prediction ellipse using the non-TA studies (Fig. S5).
RT Candida incidence
The mean RT Candida incidence among the 36 observational groups is 1.3 (95 % confidence interval; 0.9–1.8 %) (ESM file Fig. S1). This is the RT Candida benchmark. There was no significant trend in RT Candida incidence by year of study publication and a LOWESS line is presented (Fig. 2). Twenty-three of the 32 control groups of the concurrent control design studies were above this LOWESS line. The mean RT Candida incidence among the control groups was significantly higher than in the intervention groups from concurrent design SDD studies (p = 0.001; Fig. S4).
RT Candida incidence versus year of publication. Scatter plot of RT Candida incidence in observational groups (open black circles) versus year of study publication for which the linear regression was non-significant (not shown, p =0.72) and hence a LOWESS regression line is given. Also shown are the control groups from studies of non-TA methods (open blue triangles) and also concurrent control (CC) studies of SDD/SOD (closed red triangles). Note that the symbols are proportional to group size and the y axis is a logit scale
The RT Candida incidence was highest amongst the concurrent control groups of the SDD studies versus other types of component group (Fig. 3, ESM file Figs. S1–S4). The effect of membership of the various categories of component group on RT Candida was examined in GEE models of RT Candida together other group level variables (Table 2). The effect of membership of a concurrent control group of an SDD study was significant (p = 0.021). The group wide presence of candidemia risk factors (p = 0.001) and the proportion of admissions for trauma (p = 0.004) were also significant factors in the model.
Scatter plots of RT Candida incidence. Scatter plots of RT Candida incidence in component (C = control; I = intervention) groups of non-TA and non-concurrent (NCC), and concurrent control (CC) studies of SDD (antibiotic methods) of VAP prevention (blue symbols). The benchmark RT Candida is the summary mean (central vertical line) derived from the observation studies (black symbols) together with the 95 % prediction intervals (benchmark range; outer vertical lines). Studies with MV for >48 hours for <75 % and one outlier study [84] are not shown. Note that the x axis is a logit scale and that studies with a zero RT Candida can only be shown on this plot through use of the continuity correction. These data are displayed in more detail as caterpillar plots (Figs. S1–S4)
Candidemia
The incidence of candidemia was reported for 101 of the component groups (Table 1, Fig. S7). The incidence among concurrent control (p = 0.024) groups from studies of SDD was higher than that in the observational groups.
Both candidemia incidence and RT Candida incidence data were available from 19 groups from either observational studies or from studies of non-TA methods (Fig. 4a). The scatterplot presenting the bivariate relationship among these 19 groups, together with the linear regression line and a 95 % prediction ellipse, is shown using logit scales for each axis (Fig. 4a). This linear regression and prediction ellipse are in turn used to benchmark the groups from the studies of SDD (Fig. 4a–c). Whilst most of the control groups from studies of SDD with a non-concurrent (Fig. 4b) and concurrent design (Fig. 4c) are within this benchmark prediction ellipse, shift to the right and upward is apparent for the control groups in the latter plot (Fig. 4c).
Candidemia incidence versus RT Candida. a Scatter plot of candidemia incidence versus RT Candida incidence in observational groups and control groups from studies of non-TA methods (open black circles) for which both data were available. Both a linear regression (p =0.057) and a 95 % prediction ellipse based on these groups are shown. Also shown are the intervention groups from studies of non-TA methods (closed triangles). Also shown are the mean (central) and 95 % prediction lines (outer) derived for the full set of benchmark groups for both RT Candida (vertical) and candidemia (horizontal). Note that both the x and the y axes are a logit scale. b Scatter plot of candidemia incidence versus RT Candida incidence in control (closed green triangles) and intervention (open green triangles) groups from studies of non-concurrent design studies of SDD (open green triangles) for which both data were available. The linear regression, 95 % prediction ellipse and mean, and 95 % prediction lines from a are shown for reference. c Scatter plot of candidemia incidence versus RT Candida incidence in control (closed green triangles) and intervention (open red triangles) groups from studies of concurrent design studies of SDD (open red triangles) for which both data were available. The linear regression, 95 % prediction ellipse and mean, and 95 % prediction lines from a are shown for reference. Note that one outlier study [84] is not shown
Discussion
This is a meta-analysis of the incidence of respiratory tract colonization with Candida (RT Candida) among ICU patients receiving mechanical ventilation within studies in the literature. This analysis has examined the relationship between each of RT Candida and candida colonization at other sites with candidemia, as well as the relative importance of selective digestive decontamination (SDD) versus other candidate risk factors towards RT Candida. Only 45 of the studies are common to this meta-analysis and to the previous meta-analysis of candidemia [121]. This previous meta-analysis [121] was not restricted to the patient population receiving mechanical ventilation, and had included a higher proportion of studies with group-wide risk factors for candidemia (25 of the 103 studies [121]), versus only six of the 113 studies included here. As a consequence, there is a lower incidence of candidemia among the studies here versus previously [121]. However, even within this differently selected set of studies, the incidence of candidemia is again found to be higher among the control groups of SDD studies with a concurrent design than any other type of component group (Table 1).
There is a higher incidence of both Candida colonization at respiratory (RT Candida) and other sites among the control groups of SDD studies with a concurrent design versus other groups (Table 1). This higher incidence of RT Candida cannot be explained by year of publication (Fig. 2), nor in regression models that include the group-wide presence of risk factors for candidemia or proportion of trauma admissions or mode of respiratory sampling (Table 2).
There is a correlation between candidemia and RT Candida among the studies here for which data is available (Fig. 4). However, the relationship between RT Candida and candidemia is more complex than a simple linear correlation for the following reasons. Candidemia, with an incidence of approximately 1 % amongst ICU patients [121], is a rare outcome, and studies with fewer than 100 patients may have one or no cases [62, 142–147]. The relationship described here is at the group level rather than at the patient level. In relation to non-respiratory Candida colonization, the patient-level association has recently been examined in a multi-center study [148]. The relative risk for invasive candidiasis in the mechanically ventilated ICU patient population differs for throat, perineum, and urine sites of colonization, and also for different sampling time points [148]. Measures of non-respiratory Candida colonization among the studies here were poorly documented in relation to exact sites and timings, and in any case were available from less than half of the studies surveyed.
It should be noted that the clinical significance of RT Candida is unclear [149–155], and current consensus guidelines recommend against its specific treatment in the absence of either clear histological evidence for pulmonary infection, which is rare [145–147], or evidence of invasive disease [114, 115]. However, Candida colonization continues to remain of interest from both the individual and the population perspective.
At the level of the individual, the clinical significance remains unclear, with conflicting results of studies of Candida colonization of the respiratory tract among ICU patients. Some investigators have found that Candida colonization of the respiratory tract is associated with a worse outcome [149] in association with evidence of increased systemic inflammation [150]. However, a subsequent pilot study of antifungal therapy for RT Candida did not find sufficient evidence of benefit to justify proceeding to a full-scale controlled trial [151]. By contrast, other workers have not found an association with a higher mortality risk [144, 152] in ICU patients, even though there were higher disease severity scores or degree of organ dysfunction at ICU admission. Moreover, no apparent outcome benefit associated with the use of empiric systemic anti-fungal therapy in this patient group was found in either this study [152] or in a large multi-center study [153].
From the population perspective, Candida colonization is an important constituent of the ICU microbiome. RT Candida could increase the risk for co-infection with antibiotic resistant bacteria in the airway [154], through molecular interactions with bacterial pathogens [117] for which anti-fungal therapy may be protective [118].
RT Candida has potential use as a more readily available indicator for benchmarking Candida colonization incidence in the patient group receiving mechanical ventilation. RT Candida is used here for this purpose so as to benchmark Candida colonization across different studies that have examined a variety of interventions, whether using TA-based regimens such as SDD or non-TA-based methods for VAP prevention.
The effect of SDD on Candida colonization is unclear. On the one hand, SDD as a regimen comprising topical antibiotic and anti-fungal agents appears to be protective against fungal colonization, infection, and possibly even mortality [119, 120]. Indeed, the protection derived by SDD appears to outperform that obtained using azole antifungal prophylaxis in this patient group [120]. On the other hand, this protection is not apparent in the largest study, which had a non-concurrent design [155]. Moreover, SDD may have complex effects on the ICU microbiome. Indeed, in the first SDD study [74] it was asked whether this indirect effect of SDD might confound any attempt to estimate the direct effects using a conventional concurrent study design.
An uncalibrated analysis of the available Candida colonization data, whether as RT Candida or as Candida colonization not restricted to respiratory sites and not restricted to patients with suspected VAP, is consistent with what appears to be a near halving in colonization incidences, as implied in the meta-analyses of the concurrent design SDD studies [119, 120]. However, on closer scrutiny and using the RT Candida benchmark for calibration, the true impact of SDD on the incidences of Candida colonization as well as on candidemia would appear to be a near doubling amongst the concurrent control groups of SDD studies (Table 1). This occurs presumably as a result of an indirect contextual effect through inapparent cross infection [156]. By contrast, the effect of SDD on RT Candida in studies that are non-concurrent is insignificant (Table 2), as observed elsewhere [155].
It is not the intention here to examine the substantial number of different SDD regimens but rather the component groups from the two broad categories of TA and non-TA studies. In any case, it should be noted that complete Candida decolonization using SDD is difficult to achieve [157, 158]. Two recent studies of ICU patients that were colonized with Candida and were receiving SDD provided conflicting evidence that the administration of nebulised amphotericin additional to SDD might confer clinical benefit [157] versus harm [158]. Of note in both studies, the time to achieve 50 % decolonization with the addition of nebulised amphotericin to the standard SDD regime was 5 days in both studies. By contrast, among a multi-center study of 3,000 ICU patients colonized with Candida receiving routine systemic antifungal therapy with a mean ICU stay of 5 days, typically between 40 and 50 % remain colonized on ICU discharge [159].
There are four specific challenges in undertaking an analysis of RT Candida. First, the potential for transmission of Candida between control and intervention group patients in the same study renders the presumption of independence of RT Candida events untenable [160].
Second, for most SDD studies the primary end point was VAP occurrence, and RT Candida was an occasional secondary end-point. How studies with zero RT Candida events are optimally included in any analysis is important to the conclusions. Studies with zero RT Candida events should not be overlooked, as they provide potential evidence against a contextual effect. However, the majority of the SDD studies were smaller than 60 patients and, being a rare event (<2 % in most studies), a zero RT Candida event rate is unsurprising. As a consequence, the upper 95 % confidence intervals for these groups are non-trivial in caterpillar plots (Figs. S1–S4). Note that for a group of size N = 60 with zero events ,the upper 95 % confidence can be approximated by the ‘rule of three’ as 5 % (=3/N) [161].
Thirdly, to quantify a contextual effect requires a calibration to a benchmark range derived using for reference data from studies from comparable target populations. The final issue is one of validity: does RT Candida correlate with a clinically relevant and commonly reported end-point?
To deal with the first and third of these challenges, GEE-based analytic strategies have been used here to model the RT Candida of both control and intervention groups of all studies within a single analytic model as a statistical calibration (Table 2). There is an upward dispersion in RT Candida incidence among concurrent control groups from SDD studies away from this benchmark (Fig. 3). This upward dispersion is apparent in the GEE models as positive coefficients in association with membership of concurrent control groups within studies of SDD/SOD (Table 2).
To deal with the second and third issues, the continuity correction has been used to enable zero-event groups to be represented on the logit scale, which enables several types of graphical display for the purposes of a visual calibration (Figs. S1–S4; Fig. 3). Moreover, the validity issue is able to be addressed through an examination of the bivariate relationship between RT Candida and candidemia (Figs. 4a–c). The visual analysis of the bivariate relationship is aided by the use of a 95 % prediction ellipse in the plots, a method which is better suited to this purpose than linear regression [138–141]. All of these visual displays dramatically reveal that the component groups of all types, with the exception of those concurrent control groups from studies of SDD, each have a distribution similar to the observational groups from which the benchmark was derived. Strikingly, even the distribution of the intervention groups of the SDD studies are similar to those from which the benchmark was derived. This would imply that any apparent effect of SDD on Candida colonization and candidemia within concurrent control design studies is not explainable as simply a direct anti-fungal prevention effect occurring within the intervention group (Fig. S7).
There are several limitations to this study. This is an analysis at the group level, and is unable to take account of patient-specific risk factors for RT Candida. For example, the usage of empiric (non-protocolized) antifungal therapy in each study is an important unknown, as non-use may account for vulnerability to RT Candida at the individual level. However, it is unlikely that such unidentified patient-level risk factors would be able to account for the discrepancies noted here. Such a putative patient-level risk factor would need to be a consistently strong risk factor for RT Candida across all the studies and yet also be profoundly unevenly distributed, predominating in the groups of the SDD studies versus other groups within the broader evidence base examined here.
Another limitation is the imprecision associated with the diagnosis of VAP, which may lead to the potential for observer detection bias of RT Candida. That the mode of VAP diagnosis and the use of topical placebo were not significant factors in the regression model (Table 2) implies that this bias is likely to be minimal. Moreover, topical placebo use can be taken as a surrogate indicator of a study that was observer-blinded. A further limitation is the question of non-reporting of RT Candida amongst potentially eligible studies. However, the correlation between RT Candida and candidemia provides some validation, at least amongst those studies for which both data were available.
It is never possible to be certain that every relevant study has been obtained in a literature search or that the search has been truly adequate. Restricting the analysis to those studies obtained from systematic reviews attempts to provide the basis for an analysis of data derived through an independent and transparent search. That the findings of such a restricted analysis are similar to the full analysis would imply that the search has been adequate and that the number of missing studies required to alter the findings would need to be substantial.
Conclusion
The RT Candida incidence within observational groups of mechanically ventilated patients is 1.3 % (this is the RT Candida benchmark). The incidence of RT Candida and candidemia are correlated. There is insufficient information to discern how closely Candida colonization at other sites is correlated with candidemia. At the group level, the presence of candidemia risk factors, the proportion of trauma admissions, and membership of a concurrent control group within an SDD study are each risk factors for RT Candida. The apparent protection against Candida colonization from the use of SDD appears spurious, as the incidence of both RT Candida and colonization at other sites is higher among concurrent control groups of SDD studies than among observational and indeed other types of component group. These observations, as with similar observations for VAP [123], candidemia [121], and bacteremia [122] incidences among these studies, are paradoxical. Apart from major publication bias, or the effect of any major and as yet unidentified and mal-distributed patient-level risk factors for RT Candida, these profound discrepancies indicate a major contextual hazard associated with the topical antibiotic component of SDD on RT Candida within studies with concurrent controls. These increased incidences would be inapparent within individual SDD studies examined in isolation [156]. Abbreviations used in this paper are: ICU, intensive care unit; MV, mechanical ventilation ; SDD, selective digestive decontamination; VAP, ventilator-associated pneumonia; RT Candida, Candida among patients with ventilator-associated pneumonia.
References
Antonelli M, Moro ML, Capelli O et al (1994) Risk factors for early onset pneumonia in trauma patients. Chest 105:224–228
Bercault N, Boulain T (2001) Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: a prospective case–control study. Crit Care Med 29:2303–2309
Boots RJ, Phillips GE, George N, Faoagali JL (2008) Surveillance culture utility and safety using low‐volume blind bronchoalveolar lavage in the diagnosis of ventilator‐associated pneumonia. Respirology 13:87–96
Bregeon F, Papazian L, Visconti A, Gregoire R, Thirion X, Gouin F (1997) Relationship of microbiologic diagnostic criteria to morbidity and mortality in patients with ventilator-associated pneumonia. JAMA 277:655–662
Cade JF, McOwat E, Siganporia R, Keighley C, Presneill J, Sinickas V (1992) Uncertain relevance of gastric colonization in the seriously ill. Intensive Care Med 18:210–217
Cavalcanti M, Ferrer M, Ferrer R et al (2006) Risk and prognostic factors of ventilator-associated pneumonia in trauma patients. Crit Care Med 34:1067–1072
Cardenosa Cendrero JA, Sole-Violan J, Bordes Benitez A et al (1999) Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest 116:462–470
Chastre J, Trouillet JL, Vuagnat A et al (1998) Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 157:1165–1172
Chevret S, Hemmer M, Carlet J et al (1993) Incidence and risk factors of pneumonia acquired in intensive care units. Results from a multicenter prospective study on 996 patients. European Cooperative Group on Nosocomial Pneumonia. Intensive Care Med 19:256–264
Craven DE, Kunches LM, Lichtenberg DA, Kollisch NR, Barry MA, Heeren TC, McCabe WR (1988) Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med 148:1161–1168
Daschner F, Kappstein I, Schuster F et al (1988) Influence of disposable (‘Conchapak’) and reusable humidifying systems on the incidence of ventilation pneumonia. J Hosp Infect 11:161–168
de Latorre FJ, Pont T, Ferrer A et al (1995) Pattern of tracheal colonization during mechanical ventilation. Am J Respir Crit Care Med 152:1028–1033
Ensminger SA, Wright RS, Baddour LM, Afess B (2006) Suspected ventilator-associated pneumonia in cardiac patients admitted to the coronary care unit. Mayo Clin Proc 81:32–35
Ewig S, Torres A, El-Ebiary M et al (1999) Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med 159:188–198
George DL, Falk PS, Wunderink RG, Leeper KV Jr, Meduri GU, Steere EL, Glen Mayhall C (1998) Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med 158:1839–1847
Gursel G, Aydogdu M, Nadir Ozis T, Tasyurek S (2010) Comparison of the value of initial and serial endotracheal aspirate surveillance cultures in predicting the causative pathogen of ventilator-associated pneumonia. Scand J Infect Dis 42:341–346
Hugonnet S, Uçkay I, Pittet D (2007) Staffing level: a determinant of late-onset ventilator-associated pneumonia. Crit Care 11(4):R80
Ibrahim EH, Ward S, Sherman G, Kollef MH (2000) A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest 117:1434–1442
Kautzky S, Staudinger T, Presterl E (2014) Invasive Candida infections in patients of a medical intensive care unit. Wien Klin Wochenschr 127:1–11
Kollef MH, Vlasnik J, Sharpless L, Pasque C, Murphy D, Fraser V (1997) Scheduled change of antibiotic classes: A strategy to decrease the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med 156:1040–1048
Luna CM, Blanzaco D, Niederman MS et al (2003) Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med 31:676–682
Magnason S, Kristinsson KG, Stefansson T, Erlendsdottir H, Jonsdottir K, Kristjansson M, Gudmundsson S (2008) Risk factors and outcome in ICU‐acquired infections. Acta Anaesthesiol Scand 52:1238–1245
Mahul P, Auboyer C, Jospe R, Ros A, Guerin C, el Khouri Z, Galliez M, Dumont A, Gaudin O (1992) Prevention of nosocomial pneumonia in intubated patients respective role of mechanical subglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med 18:20–25
Memish ZA, Cunningham G, Oni GA et al (2000) The incidence and risk factors of ventilator-associated pneumonia in a Riyadh hospital. Infect Control Hosp Epidemiol 21:271–273
Moine P, Timsit JF, De Lassence A et al (2002) Mortality associated with late-onset pneumonia in the intensive care unit: results of a multi-center cohort study. Intensive Care Med 28:154–163
Potgieter PD, Linton DM, Oliver S, Forder AA (1987) Nosocomial infections in a respiratory intensive care unit. Crit Care Med 15:495–498
Rello J, Quintana E, Ausina V et al (1991) Incidence, etiology, and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest 100:439–444
Rello J, Ausina V, Castella J et al (1992) Nosocomial respiratory tract infections in multiple trauma patients. Influence of level of consciousness with implications for therapy. Chest 102:525–529
Rello J, Lorente C, Diaz E et al (2003) Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest 124:2239–2243
Resende MM, Monteiro SG, Callegari B, Figueiredo PM, Monteiro CR, Monteiro-Neto V (2013) Epidemiology and outcomes of ventilator-associated pneumonia in northern Brazil: an analytical descriptive prospective cohort study. BMC Infect Dis 13(1):119
Reusser P, Zimmerli W, Scheidegger D, Marbet GA, Buser M, Gyr K (1989) Role of gastric colonization in nosocomial infections and endotoxemia: a prospective study in neurosurgical patients on mechanical ventilation. J Infect Dis 160:414–421
Ruiz-Santana S, Garcia Jimenez A, Esteban A et al (1987) ICU pneumonias: a multi-institutional study. Crit Care Med 15:930–932
Salata RA, Lederman MM, Shlaes DM, Jacobs MR, Eckstein E, Tweardy D, Toossi Z, Chmielewski R, Marino J, King CH (1987) Diagnosis of nosocomial pneumonia in intubated, intensive care unit patients. Am Rev Respir Dis 135:426–432
Shahin J, Bielinski M, Guichon C, Flemming C, Kristof AS (2013) Suspected ventilator-associated respiratory infection in severely ill patients: a prospective observational study. Crit Care 17(5):R251
Xie DS, Xiong W, Lai RP, Liu L, Gan XM, Wang XH, Nie SF (2011) Ventilator-associated pneumonia in intensive care units in Hubei Province, China: a multicentre prospective cohort survey. J Hosp Infect 78(4):284–288
Acosta-Escribano J, Fernández-Vivas M, Carmona TG, Caturla-Such J, Garcia-Martinez M, Menendez-Mainer A, Sanchez-Payá J (2010) Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intensive Care Med 36:1532–1539
Bellissimo-Rodrigues WT, Menegueti MG, Gaspar GG, Nicolini EA, Auxiliadora-Martins M, Basile-Filho A, Bellissimo-Rodrigues F (2014) Effectiveness of a dental care intervention in the prevention of lower respiratory tract nosocomial infections among intensive care patients: a randomized clinical trial. Infect Control Hosp Epidemiol 35:1342–1348
Caruso P, Denari S, Ruiz SA, Demarzo SE, Deheinzelin D (2009) Saline instillation before tracheal suctioning decreases the incidence of ventilator-associated pneumonia. Crit Care Med 37:32–38
Cook D, Guyatt G, Marshall J et al (1998) A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med 338:791–797
Daumal F, Colpart E, Manoury B, Mariani M, Daumal M (1999) Changing heat and moisture exchangers every 48 hours does not increase the incidence of nosocomial pneumonia. Infect Control 20(05):347–349
Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogué S, Ferrer M (1999) Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 354(9193):1851–1858
Driks MR, Craven DE, Celli BR et al (1987) Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization. N Engl J Med 317:1376–1382
Fourrier FE, Cau-Pottier H, Boutigny M, Roussel-Delvallez M, Jourdain CC (2000) Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Med 26:1239–1247
Fourrier F, Dubois D, Pronnier P, Herbecq P, Leroy O, Desmettre T, Roussel-Delvallez M (2005) Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit a double-blind placebo-controlled multicenter study. Crit Care Med 33:1728–1735
Giglio M, Caggiano G, Dalfino L, Brienza N, Alicino I, Sgobio A, Favale A, Puntillo F (2012) Oral nystatin prophylaxis in surgical/trauma ICU patients: a randomised clinical trial. Crit Care 16(2):R57
Heyland DK, Cook DJ, Schoenfeld PS, Frietag A, Varon J, Wood G (1999) The effect of acidified enteral feeds on gastric colonization in critically ill patients: results of a multicenter randomized trial. Canadian Critical Care Trials Group. Crit Care Med 27:2399–2406
Holzapfel L, Chevret S, Madinier G, Ohen F, Demingeon G, Coupry A, Chaudet M (1993) Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med 21:1132–1138
Holzapfel L, Chastang C, Demingeon G, Bohe J, Piralla B, Coupry A (1999) A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am J Respir Crit Care Med 159:695–701
Koeman M, van der Ven AJ, Hak E et al (2006) Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med 173:1348–1355
Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, Schinner R (2008) Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA 300(7):805–813
Lacherade JC, Auburtin M, Cerf C et al (2005) Impact of humidification systems on ventilator-associated pneumonia: a randomized multicenter trial. Am J Respir Crit Care Med 172:1276–1282
Lorente L, Lecuona M, Malaga J et al (2003) Bacterial filters in respiratory circuits: an unnecessary cost? Crit Care Med 31:2126–2130
Lorente L, Lecuona M, Galvan R et al (2004) Periodically changing ventilator circuits is not necessary to prevent ventilator-associated pneumonia when a heat and moisture exchanger is used. Infect Control Hosp Epidemiol 25:1077–1082
Lorente L, Lecuona M, Martin MM et al (2005) Ventilator-associated pneumonia using a closed versus an open tracheal suction system. Crit Care Med 33:115–119
Lorente L, Lecuona M, Jimenez A et al (2006) Tracheal suction by closed system without daily change versus open system. Intensive Care Med 32:538–544
Lorente L, Lecuona M, Jimenez A, Mora ML, Sierra A (2006) Ventilator-associated pneumonia using a heated humidifier or a heat and moisture exchanger: a randomized controlled trial [ISRCTN88724583]. Crit Care 10:R116
Lorente L, Lecuona M, Jimenez A, Mora ML, Sierra (2007) Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med 176:1079–1083
Lorente L, Lecuona M, Jiménez A, Palmero S, Pastor E, Lafuente N, Ramos MJ, Mora ML, Sierra A (2012) Ventilator-associated pneumonia with or without toothbrushing a randomized controlled trial. Eur J Clin Microbiol Infect Dis 31:1–9
Martin LF, Booth FV, Karlstadt RG et al (1993) Continuous intravenous cimetidine decreases stress-related upper gastrointestinal hemorrhage without promoting pneumonia. Crit Care Med 21:19–30
Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M (2006) Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med 32(2):230–236
Morrow LE, Kollef MH, Casale TB (2010) Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med 182:1058–1064
Normand S, François B, Dardé ML, Bouteille B, Bonnivard M, Preux PM, Vignon P (2005) Oral nystatin prophylaxis of Candida spp. colonization in ventilated critically ill patients. Intensive Care Med 31(11):1508–1513
Pickworth KK, Falcone RE, Hoogeboom JE et al (1993) Occurrence of nosocomial pneumonia in mechanically ventilated trauma patients: a comparison of sucralfate and ranitidine. Crit Care Med 21:1856–1862
Pneumatikos I, Konstantonis D, Tsagaris I et al (2006) Prevention of nosocomial maxillary sinusitis in the ICU: the effects of topically applied alpha-adrenergic agonists and corticosteroids. Intensive Care Med 32:532–537
Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, Bas A (2006) Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 296(20):2460–2466
Sirvent JM, Torres A, El-Ebiary M, Castro P, de Batlle J, Bonet A (1997) Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med 155:1729–1734
Smulders K, van der Hoeven H, Weers-Pothoff I, Vandenbroucke-Grauls C (2002) A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest 121:858–862
Staudinger T, Bojic A, Holzinger U, Meyer B, Rohwer M, Mallner F, Locker GJ (2010) Continuous lateral rotation therapy to prevent ventilator-associated pneumonia. Crit Care Med 38(2):486–490
Thomachot L, Viviand X, Arnaud S, Boisson C, Martin CD (1998) Comparing two heat and moisture exchangers, one hydrophobic and one hygroscopic, on humidifying efficacy and the rate of nosocomial pneumonia. Chest 114:1383–1389
Thomachot L, Vialet R, Arnaud S et al (1999) Do the components of heat and moisture exchanger filters affect humidifying efficacy and the incidence of nosocomial pneumonia? Crit Care Med 27:923–928
Bergmans DC, Bonten MJ, Gaillard CA et al (2001) Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 164:382–388
Camus C, Salomon S, Bouchigny C, Gacouin A, Lavoué S, Donnio PY, Bellissant E (2014) Short-term decline in all-cause acquired infections with the routine use of a decontamination regimen combining topical polymyxin, tobramycin, and amphotericin B with mupirocin and chlorhexidine in the ICU: a single-center experience. Crit Care Med 42:1121–1130
Hartenauer U, Thulig B, Diemer W, Lawin P, Fegeler W, Kehrel R, Ritzerfeld W (1991) Effect of selective flora suppression on colonization, infection, and mortality in critically ill patients: a one-year, prospective consecutive study. Crit Care Med 19:463–473
Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF, Langrehr D (1987) The effect of oropharyngeal decontamination using topical nonabsorbable antibiotics on the incidence of nosocomial respiratory tract infections in multiple trauma patients. J Trauma 27:357–364
Winter R, Humphreys H, Pick A et al (1992) A controlled trial of selective decontamination of the digestive tract in intensive care and its effect on nosocomial infection. J Antimicrob Chemother 30:73–87
David A (2007) Infektionen und Keimspektrum nach einer Dekade der selektiven Darmdekontamination (SDD) in der operativen Intensivmedizin. [Infections and germ spectrum after a decade of selective intestinal decontamination (SDD) in surgical intensive care medicine] Dissertation, Universitätsklinikum Münster. http://nbn-resolving.de/urn:nbn:de:hbz:6–08599456972. Accessed 10 November 2015
Hjortrup A, Rasmussen A, Hansen BA, Hoiby N, Heslet L, Moesgaard F, Kirkegaard P (1997) Early bacterial and fungal infections in liver transplantation after oral selective bowel decontamination. Transplant Proc 29:3106–3110
Nardi G, Di Silvestre A, De Monte A, Massarutti D, Proietti A, Troncon MG, Zussino M (2001) Reduction in gram-positive pneumonia and antibiotic consumption following the use of a SDD protocol including nasal and oral mupirocin. Eur J Emerg Med 8:203–214
Silvestri L, Bragadin CM, Milanese M, Gregori D, Consales C, Gullo A, van Saene HFK (1999) Are most ICU infections really nosocomial? A prospective observational cohort study in mechanically ventilated patients. J Hosp Infect 42:125–133
Steffen R, Reinhartz O, Blumhardt G, Bechstein WO, Raakow R, Langrehr JM, Neuhaus P (1994) Bacterial and fungal colonization and infections using oral selective bowel decontamination in orthotopic liver transplantations. Transpl Int 7(2):101–108
Aerdts SJ, van Dalen R, Clasener HA, Festen J, van Lier HJ, Vollaard EJ (1991) Antibiotic prophylaxis of respiratory tract infection in mechanically ventilated patients. A prospective, blinded, randomized trial of the effect of a novel regimen. Chest 100:783–791
Bion JF, Badger I, Crosby HA, Hutchings P, Kong KL, Baker J, Hutton P, McMaster P, Buckels JA, Elliott TSJ (1994) Selective decontamination of the digestive tract reduces gram-negative pulmonary colonization but not systemic endotoxemia in patients undergoing elective liver transplantation. Crit Care Med 22:40–49
Blair P, Rowlands BJ, Lowry K, Webb H, Armstrong P, Smilie J (1991) Selective decontamination of the digestive tract: a stratified, randomized, prospective study in a mixed intensive care unit. Surgery 110:303–309
Cerra FB, Maddaus MA, Dunn DL, Wells CL, Konstantinides NN, Lehmann SL, Mann HJ (1992) Selective gut decontamination reduces nosocomial infections and length of stay but not mortality or organ failure in surgical intensive care unit patients. Arch Surg 127:163–167
de La Cal MA, Cerda E, Garcia-Hierro P, Van Saene HK, Gómez-Santos D, Negro E, Lorente JA (2005) Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo-controlled, double-blind trial. Ann Surg 241:424–430
Ferrer M, Torres A, Gonzalez J, Puig de la Bellacasa J, el-Ebiary M, Roca M, Gatell JM, Rodriguez-Roisin R (1994) Utility of selective digestive decontamination in mechanically ventilated patients. Ann Intern Med 120:389–395
Flaherty J, Nathan C, Kabins SA, Weinstein RA (1990) Pilot trial of selective decontamination for prevention of bacterial infection in an intensive care unit. J Infect Dis 162:1393–1397
Gaussorgues P, Salord M, Sirodot S, Tigaud S, Cagnin S, Gerard M, Robert D (1991) Efficiency of selective decontamination of the digestive tract on the occurrence of nosocomial bacteremia in patients on mechanical ventilation receiving betamimetic therapy. Réan Soins Intens Méd Urg 7:169–174
Georges B, Mazerolles M, Decun J-F, Rouge P, Pomies S, Cougot P (1994) Décontamination digestive sélective résultats d'une étude chez le polytraumatisé. Réan Soins Intens Méd Urg 3:621–627
Hünefeld G (1988) A clinical study of selective gut decolonization in 204 long-term ventilated intensive care patients undergoing abdominal and accident surgery. Anaesthesiol Reanim 14:131–153
Jacobs S, Foweraker JE, Roberts SE (1992) Effectiveness of selective decontamination of the digestive tract (SDD) in an ICU with a policy encouraging a low gastric pH. Clin Intensive Med 3:52–58
Kerver AJ, Rommes JH, Mevissen-Verhage EA, Hulstaert PF, Vos A, Verhoef J, Wittebol P (1988) Prevention of colonization and infection in critically ill patients: a prospective randomized study. Crit Care Med 16:1087–1093
Kollef M, Pittet D, Sanchez Garcia M et al (2006) A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med 173:91–97
Korinek AM, Laisne MJ, Nicolas MH, Raskine L, Deroin V, Sanson-Lepors MJ (1993) Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: a double-blind, randomized, placebo-controlled study. Crit Care Med 21:1466–1473
Laggner AN, Tryba M, Georgopoulos A, Lenz K, Grimm G, Graninger W, Schneeweiss B, Druml W (1994) Oropharyngeal decontamination with gentamicin for long-term ventilated patients on stress ulcer prophylaxis with sucralfate? Wien Klin Wochenschr 106:15–19
Palomar M, Alvarez-Lerma F, Jorda R, Bermejo B, Catalan Study Group of Nosocomial Pneumonia Prevention (1997) Prevention of nosocomial infection in mechanically ventilated patients: selective digestive decontamination versus sucralfate. Clin Intensive Care 8:228–235
Pneumatikos I, Koulouras V, Nathanail C, Goe D, Nakos G (2002) Selective decontamination of subglottic area in mechanically ventilated patients with multiple trauma. Intensive Care Med 28:432–437
Quinio B, Albanese J, Bues-Charbit M, Viviand X, Martin C (1996) Selective decontamination of the digestive tract in multiple trauma patients. A prospective double-blind, randomized, placebo-controlled study. Chest 109:765–772
Rocha LA, Martin MJ, Pita S, Paz J, Seco C, Margusino L, Villanueva R, Duran MT (1992) Prevention of nosocomial infection in critically ill patients by selective decontamination of the digestive tract. A randomized, double blind, placebo-controlled study. Intensive Care Med 18:398–404
Rolando N, Gimson A, Wade J, Philpott‐Howard J, Casewell M, Williams R (1993) Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 17:196–201
Rolando N, Wade JJ, Stangou A, Gimson AE, Wendon J, Philpott‐Howard J, Williams R (1996) Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg 2:8–13
Sanchez Garcia M, Cambronero Galache JA, Lopez Diaz J, Cerda Cerda E, Rubio Blasco J, Gomez Aguinaga MA, Nunez Reiz A, Rogero Marin S, Onoro Canaveral JJ, Sacristan del Castillo JA (1998) Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled, multicenter trial. Am J Respir Crit Care Med 158:908–916
Smith SD, Jackson RJ, Hannakan CJ, Wadowsky RM, Tzakis AG, Rowe MI (1993) Selective decontamination in pediatric liver transplants. Transplantation 55:1306–1308
Stoutenbeek CP, van Saene HKF, Little RA, Whitehead A (2007) The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: a multicenter randomized controlled trial. Intensive Care Med 33:261–270
Tetteroo GWM, Castelein A, Tilanus HW, Ince C, Bruining HA, Wagenvoort JHT (1990) Selective decontamination to reduce gram-negative colonisation and infections after oesophageal resection. Lancet 335:704–707
Ulrich C, Harinck-de Weerd JE, Bakker NC, Jacz K, Doornbos L, de Ridder VA (1989) Selective decontamination of the digestive tract with norfloxacin in the prevention of ICU-acquired infections: a prospective randomized study. Intensive Care Med 15:424–431
Unertl K, Ruckdeschel G, Selbmann HK et al (1987) Prevention of colonization and respiratory infections in long-term ventilated patients by local antimicrobial prophylaxis. Intensive Care Med 13:106–113
Verwaest C, Verhaegen J, Ferdinande P, Schetz M, Van den Berghe G, Verbist L, Lauwers P (1997) Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 25:63–71
Wiener J, Itokazu G, Nathan C, Kabins SA, Weinstein RA (1995) A randomized, double-blind, placebo-controlled trial of selective digestive decontamination in a medical–surgical intensive care unit. Clin Infect Dis 20:861–867
Zobel G, Kuttnig M, Grubbauer HM, Semmelrock HJ, Thiel W (1991) Reduction of colonization and infection rate during pediatric intensive care by selective decontamination of the digestive tract. Crit Care Med 19:1242–1246
Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D (2002) Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med 28:1708–1717
Garbino J, Pichard C, Pichna P, Pittet D, Lew D, Romand J (2004) Impact of enteral versus parenteral nutrition on the incidence of fungal infections: a retrospective study in ICU patients on mechanical ventilation with selective digestive decontamination. Clin Nutr 23:705–710
Verhaegen J (1992) Randomized study of selective digestive decontamination on colonization and prevention of infection in mechanically ventilated patients in the ICU. Dissertation, University Hospital, Leuven, Belgium
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Ullmann AJ (2012) ESCMID guideline for the diagnosis and management of Candida diseases 2012: non‐neutropenic adult patients. Clin Microbiol Infect 18(s7):19–37
Torres A, Ewig S, Lode H, Carlet J, European HAP Working Group (2009) Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med 35(1):9–29
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Lindsay AK, Hogan DA (2015) Candida albicans: molecular interactions with Pseudomonas aeruginosa and Staphylococcus aureus. Fungal Biol Rev 28:85–96
Nseir S, Jozefowicz E, Cavestri B, Sendid B, Di Pompeo C, Dewavrin F, Durocher A (2007) Impact of antifungal treatment on Candida–Pseudomonas interaction: a preliminary retrospective case–control study. Intensive Care Med 33(1):137–142
Silvestri L, Van Saene HK, Milanese M, Gregori D (2005) Impact of selective decontamination of the digestive tract on fungal carriage and infection: systematic review of randomized controlled trials. Intensive Care Med 31:898–910
van Till JO, van Ruler O, Lamme B, Weber RJ, Reitsma JB, Boermeester MA (2007) Single-drug therapy or selective decontamination of the digestive tract as antifungal prophylaxis in critically ill patients: a systematic review. Crit Care 11:R126
Hurley JC (2015) ICU-acquired candidemia within selective digestive decontamination studies: a meta-analysis. Intensive Care Med 41:1877–1885
Hurley JC (2014) Topical antibiotics as a major contextual hazard toward bacteremia within selective digestive decontamination studies: a meta-analysis. BMC Infect Dis 14:714
Hurley JC (2014) Ventilator-associated pneumonia prevention methods using topical antibiotics: herd protection or herd peril? Chest 146(4):890–898
Melsen WG, Rovers MM, Bonten MJM (2009) Ventilator-associated pneumonia and mortality: A systematic review of observational studies. Crit Care Med 37:2709–2718
Safdar N, Dezfulian C, Collard HR, Saint S (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 33:2184–2193
Agrafiotis M, Siempos II, Ntaidou TK, Falagas ME (2011) Attributable mortality of ventilator-associated pneumonia: a meta-analysis. Int J Tuberc Lung Dis 15:1154–1163
Messori A, Trippoli S, Vaiani M, Gorini M, Corrado A (2000) Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: meta-analysis of randomised controlled trials. BMJ 321:1103–1106
Huang J, Cao Y, Liao C, Wu L, Gao F (2010) Effect of histamine-2-receptor antagonists versus sucralfate on stress ulcer prophylaxis in mechanically ventilated patients: a meta-analysis of 10 randomized controlled trials. Crit Care 14:R194
Alhazzani W, Almasoud A, Jaeschke R, Lo BW, Sindi A, Altayyar S, Fox-Robichaud A (2013) Small bowel feeding and risk of pneumonia in adult critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care 17:R127
Chan EY, Ruest A, Meade MO, Cook DJ (2007) Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 334:889–900
Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI (2011) Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis 11:845–854
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 4
Vandenbroucke-Grauls CM, Vandenbroucke JP (1991) Effect of selective decontamination of the digestive tract on respiratory tract infections and mortality in the intensive care unit. Lancet 338:859–862
Hurley JC (1995) Prophylaxis with enteral antibiotics in ventilated patients: selective decontamination or selective cross-infection? Antimicrob Agents Chemother 39:941–947
Pileggi C, Bianco A, Flotta D, Nobile CG, Pavia M (2011) Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care 15:R155
Hurley JC (2008) Profound effect of study design factors on ventilator-associated pneumonia incidence of prevention studies: benchmarking the literature experience. J Antimicrob Chemother 61:1154–1161
Hurley JC (2011) Paradoxical ventilator-associated pneumonia incidences among selective digestive decontamination studies versus other studies of mechanically ventilated patients: benchmarking the evidence base. Crit Care 15:R7
Alexandersson A (1998) gr32: Confidence ellipses. Stata Technical Bulletin 46: 10–13. In: Stata Technical Bulletin Reprints, vol 8. Stata Press, College Station, pp 54–57
Alexandersson A (2004) Graphing confidence ellipses: An update of ellip for Stata 8. Stata J 4:242–256
Friendly M, Monette G, Fox J (2013) Elliptical insights: understanding statistical methods through elliptical geometry. Stat Sci 28:1–39
Cleveland WS (1979) Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74:829–836
Charles PE, Dalle F, Aube H, Doise JM, Quenot JP, Aho LS, Blettery B (2005) Candida spp. colonization significance in critically ill medical patients: a prospective study. Intensive Care Med 31:393–400
Rello J, Esandi ME, Diaz E, Mariscal D, Gallego M, Vallès J (1998) The role of Candida sp isolated from bronchoscopic samples in nonneutropenic patients. Chest 114(1):146–149
Wood GC, Mueller EW, Croce MA, Boucher BA, Fabian TC (2006) Candida sp. isolated from bronchoalveolar lavage: clinical significance in critically ill trauma patients. Intensive Care Med 32(4):599–603
El-Ebiary M, Torres A, Fabregas N, de la Bellacasa JP, Gonzalez J, Ramirez J, Jiménez de Anta MT (1997) Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients: an immediate postmortem histologic study. Am J Respir Crit Care Med 156(2):583–590
Schnabel RM, Linssen CF, Guion N, van Mook WN, Bergmans DC (2014) Candida pneumonia in intensive care unit? Open Forum Infect Dis 1(1):ofu026
Meersseman W, Lagrou K, Spriet I, Maertens J, Verbeken E, Peetermans WE, Van Wijngaerden E (2009) Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 35(9):1526–1531
Lau AF, Kabir M, Chen SCA, Playford EG, Marriott DJ, Jones M, Sorrell TC (2015) Candida colonization as a risk marker for invasive candidiasis in mixed medical-surgical intensive care units: Development and evaluation of a simple, standard protocol. J Clin Microbiol 53:1324–1330
Delisle MS, Williamson DR, Perreault MM, Albert M, Jiang X, Heyland DK (2008) The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J Crit Care 23(1):11–17
Williamson DR, Albert M, Perreault MM, Delisle MS, Muscedere J, Rotstein C, Heyland DK (2011) The relationship between Candida species cultured from the respiratory tract and systemic inflammation in critically ill patients with ventilator-associated pneumonia. Can J Anesth/J Can Anesth 58(3):275–284
Albert M, Williamson D, Muscedere J, Lauzier F, Rotstein C, Kanji S, Heyland D (2014) Candida in the respiratory tract secretions of critically ill patients and the impact of antifungal treatment: a randomized placebo controlled pilot trial (CANTREAT study). Intensive Care Med 40(9):1313–1322
Terraneo S, Ferrer M, Martín-Loeches I, Esperatti M, Di Pasquale M, Giunta V, Rinaudo M, de Rosa F, Li Bassi G, Centanni S, Torres A (2016) Impact of Candida spp. isolation in the respiratory tract in patients with intensive care unit-acquired pneumonia. Clin Microbiol Infect 22(1):94.e1–94.e8
Bailly S, Bouadma L, Azoulay E, Orgeas MG, Adrie C, Souweine B, Timsit JF (2015) Failure of empirical systemic antifungal therapy in mechanically ventilated critically ill patients. Am J Respir Crit Care Med 191(10):1139–1146
Hamet M, Pavon A, Dalle F, Pechinot A, Prin S, Quenot JP, Charles PE (2012) Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med 38(8):1272–1279
de Smet AMGA, Kluytmans JAJW, Cooper BS, Mascini EM, Benus RFJ, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
Hurley JC (2005) Inapparent outbreaks of ventilator-associated pneumonia: an ecologic analysis of prevention and cohort studies. Infect Control Hosp Epidemiol 26:374–390
Ong DS, Klouwenberg PMK, Spitoni C, Bonten MJ, Cremer O (2013) Nebulised amphotericin B to eradicate Candida colonisation from the respiratory tract in critically ill patients receiving selective digestive decontamination: a cohort study. Crit Care 17:R233
Van der Geest PJ, Dieters EI, Rijnders B, Groeneveld JA (2014) Safety and efficacy of amphotericin-B deoxycholate inhalation in critically ill patients with respiratory Candida spp. colonization: a retrospective analysis. BMC Infect Dis 14:575
Ferreira D, Grenouillet F, Blasco G, Samain E, Hénon T, Dussaucy A, Pili-Floury S (2015) Outcomes associated with routine systemic antifungal therapy in critically ill patients with Candida colonization. Intensive Care Med 41:1077–1088
Mutapi F, Roddam A (2002) P values for pathogens: statistical inference from infectious-disease data. Lancet Infect Dis 2:219–230
Hanley JA, Lippman-Hand A (1983) If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 249:1743–1745
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Sources of funding
This work was supported by the Australian Government Department of Health and Ageing through the Rural Clinical Training and Support (RCTS) program. The funding agency had no role in the preparation of the manuscript nor its approval for submission.
Disclosure of potential conflicts of interest
The author declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by the author.
Competing interests
James Hurley declares that he has no competing interests.
Author contributions
As sole author, JH produced the design of the study, performed the statistical analysis and wrote the manuscript. JH read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
MOESM 1
ESM file: RT Candida data for observational studies (Table S1), studies of non-TA-based methods of VAP prevention (Table S2), studies of SDD with non-concurrent groups (Table S3) and studies of SDD with concurrent groups (Table S4). Caterpillar and other plots of RT Candida data (Figs. S1–S7). (PDF 517 kb)
Rights and permissions
About this article
Cite this article
Hurley, J.C. Impact of selective digestive decontamination on respiratory tract Candida among patients with suspected ventilator-associated pneumonia. A meta-analysis. Eur J Clin Microbiol Infect Dis 35, 1121–1135 (2016). https://doi.org/10.1007/s10096-016-2643-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2643-7