Abstract
Despite the increasing evidence of the benefit of corticosteroids for the treatment of moderate-severe coronavirus disease 2019 (COVID-19) patients, no data are available about the potential role of high doses of steroids for these patients. We evaluated the mortality, the risk of need for mechanical ventilation (MV), or death and the risk of developing a severe acute respiratory distress syndrome (ARDS) between high (HD) and standard doses (SD) among patients with a severe COVID-19. All consecutive confirmed COVID-19 patients admitted to a single center were selected, including those treated with steroids and an ARDS. Patients were allocated to the HD (≥ 250 mg/day of methylprednisolone) of corticosteroids or the SD (≤ 1.5 mg/kg/day of methylprednisolone) at discretion of treating physician. Five hundred seventy-three patients were included: 428 (74.7%) men, with a median (IQR) age of 64 (54–73) years. In the HD group, a worse baseline respiratory situation was observed and male gender, older age, and comorbidities were significantly more common. After adjusting by baseline characteristics, HDs were associated with a higher mortality than SD (adjusted OR 2.46, 95% CI 1.59–3.81, p < 0.001) and with an increased risk of needing MV or death (adjusted OR 2.35, p = 0.001). Conversely, the risk of developing a severe ARDS was similar between groups. Interaction analysis showed that HD increased mortality exclusively in elderly patients. Our real-world experience advises against exceeding 1–1.5 mg/kg/day of corticosteroids for severe COVID-19 with an ARDS, especially in older subjects. This reinforces the rationale of modulating rather than suppressing immune responses in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel emergent virus that was first recognized in Wuhan, China, and has since rapidly spread around the world [1].
Although most patients present a mild-moderate disease, almost one-third of patients are at high risk of developing a more severe disease due to an acute respiratory distress syndrome (ARDS) that may lead to the need for mechanical ventilation (MV) and admission to an intensive care unit, or even death [2]. The underlying mechanisms of severe COVID-19 are related to systemic inflammatory responses that can lead to lung injury and multisystem organ dysfunction [2, 3]. Based on this assumption, systemic anti-inflammatory drugs have been proposed as an alternative treatment tool to avoid the SARS-CoV-2-induced inflammatory state and to reduce mortality in these patients [3,4,5,6]. A first randomized controlled, clinical trial showed evidence that dexamethasone given at moderate doses for a short period of time reduced mortality versus usual care in hospitalized COVID-19 patients [7]. Nevertheless, there is no evidence to clarify whether higher doses may improve or worsen the outcomes, and many uncertainties remain unclear in this regard. In this scenario, we aimed to evaluate the efficacy of high doses (HDs) of corticosteroids in regard to standard doses (SDs) in hospitalized patients with COVID-19 who developed an ARDS.
Materials and methods
Study design
This single-center, retrospective, observational study was performed at Hospital Universitario Ramón y Cajal (HRC) in Madrid, the region of Spain with the highest incidence of confirmed cases of COVID-19 in March and April 2020. All adult patients admitted to HRC with highly suspected SARS-CoV-2 infection from the beginning of the pandemics to April 15, 2020, were initially selected. Eligible patients included all hospitalized adults with a positive, laboratory-confirmed test for SARS-CoV-2 developing an ARDS, treated with steroids, for whom a predefined minimum dataset was available. The minimum dose for inclusion was methylprednisolone-equivalent dosages of at least 0.5 mg/kg for 2 or more consecutive days. Patients without ARDS or treated during admission with remdesivir, a current anti-viral treatment with a potential effect over mortality in SARS-CoV-2 infection [8], were excluded.
The study was approved by the institutional ethics board of HRC. The need for informed consent from individual patients was waived due to its retrospective design.
Data collection
Trained physicians reviewed electronic medical records and extracted data for the period between admission to discharge, death, or June 22, 2020, whichever occurred first. Demographical, clinical, radiological, and laboratory information were recorded, including comorbidities, respiratory variables, and details of treatments administered for COVID-19. Two different groups of patients were established, depending on the dosages of steroids administered for ARDS:
-
HD of corticosteroids: short-term pulse therapy of methylprednisolone-equivalent dosages from 250 to 1000 mg/day during one or more consecutive days.
-
SD of corticosteroids (according to reference [9]): methylprednisolone-equivalent dosages ranging from and including 0.5 to 1.5 mg/kg/day.Footnote 1
The decision of treatment with one dosage or another was exclusively at the discretion of treating medical team, as evidence about the use of corticosteroids on COVID-19 was very low. Whenever a patient was treated with both range of doses, the allocation to one group or another was established selecting the doses administered before the outcome variable occurred (e.g., a patient initially treated with SD developing the outcome who later received a HD was considered SD). Whenever both dosages were administered before the outcome, patients were classified as HD.
The primary endpoint was the mortality between HD and SD patients. Secondary endpoints included (1) a combined variable of need for mechanical or non-invasive MV, and death and (2) the development of severe ARDS, according to the Berlin Definition [10].
Statistical analysis
Continuous variables were reported as mean (standard deviation) or median (interquartile range) depending on the distribution of data and were evaluated with a two-sample t test or the Wilcoxon rank-sum test, as appropriate. Categorical variables were described using absolute and relative frequencies and analyzed with a χ2 test. We conducted both unadjusted and multivariable logistic regression models to investigate the effect of both dosages on primary and secondary endpoints. The multivariable model was adjusted by potential confounding factors identified at baseline (gender, age-adjusted Charlson Comorbidity Index—CCI [11]—and peripheral oxygen saturation/fraction of inspired oxygen—SpO2/FiO2—ratio). To avoid reverse causality associations between treatments and outcomes, we excluded for analysis those patients receiving the first dose of corticosteroids the same day or after the endpoint of interest. Besides, in order to increase specificity by considering a sufficient effect of corticosteroids, only patients exposed to treatment at least 3 days before the event of interest were considered for analyses. We also performed sensitivity analyses to validate the strength of findings. Results from all multivariable analyses are reported as odds ratios (ORs) with 95% confidence intervals (CIs). We further analyzed interaction between both groups and age. All analyses were conducted using Stata® 14 (StataCorp, College Station, TX, USA) and were two-tailed, with P < 0.05 as the level of significance.
Results
Patient characteristics
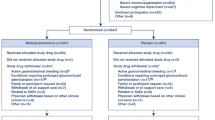
Eight hundred and thirty-six consecutively hospitalized patients with a highly suspected SARS-CoV-2 infection were screened for inclusion. We first removed patients with a negative RT-PCR assay (n = 48) or incomplete follow-up data (n = 4). After excluding patients not receiving steroids (n = 118), not developing ARDS (n = 56), or those treated with remdesivir (n = 37), 573 were finally included for analysis (Fig. 1). By far, the most chosen corticosteroid was methylprednisolone, administered to 568 (99.1%) patients. Three hundred and ninety-six patients were in the SD group (69.1%), while 177 (30.9%) were treated with higher doses (HDs). Further information about dosages, frequencies, and duration of corticosteroids regimens is provided in Supplemental Data (Table 1). Median (IQR) time from onset of symptoms to admission, to ARDS, and to first dose of steroids were 7 (4–9), 8 (6–11), and 10 (7–14) days, respectively, with no differences between both groups. Patients were followed for a median (IQR) of 21 (15–32) days. Baseline demographics, clinical data, and radiological and laboratory findings are shown in Table 1. The median age of the study population was 64 years, and HD patients were older compared to SD patients (67 versus 64 years, respectively, p = 0.03). There were significantly fewer men in the SD cohort (70.2%) than in the HD group (84.8%) (p < 0.001). Relevant comorbidities were frequent in both groups. The age-adjusted CCI was mildly higher among HD patients (median [IQR] of 3 [2–5]) compared to SD patients (median [IQR] of 3 [1–4]) (p = 0.009). In addition, patients treated with HD of steroids at the onset of ARDS presented a worse respiratory situation, with a lower SpO2/FiO2 ratio compared to SD patients (271 versus 277, respectively, p = 0.011). Baseline characteristics of patients treated with corticosteroids at least 3 days before MV (n = 396) and severe ARDS (n = 210), selected for analysis of secondary endpoints, are shown in Supplemental Data (Tables 2 and 3).
During admission, several treatments were administered to patients, being hydroxychloroquine the most frequent (98.1%), followed by lopinavir/ritonavir (92.5%), and antibiotics (89.2%), with no significant differences between both groups. Tocilizumab, a monoclonal antibody inhibiting interleukin-6 (IL-6) receptor, was equally given to both groups (40.9% among the SD cohort and 42.4% among the HD group). Only when considering rarely administered treatments, significant differences between HD and SD were observed (more anakinra and less interferon-β 1b among the HD group) (Table 4 of Supplemental Data).
Outcomes
A final admission outcome (discharge or death) was recorded in 564 out of 573 (98.4%) patients. Overall mortality after a median (IQR) of 16 (9–26) days of admission was 24.8% (n = 140), and was twice more frequently in the HD cohort than in the SD group (39% versus 18.6%, respectively). The unadjusted logistic regression model showed a significantly higher risk of death for patients with an ARDS receiving HD of corticosteroids compared to patients treated with SD (OR 2.79, 95% CI 1.87–4.15, p < 0.001). After adjusting by gender, age-adjusted CCI, and SpO2/FiO2, results remained similar (adjusted OR 2.46, 95% CI 1.59–3.81, p < 0.001). Though a shorter time from first dose of corticosteroid to death (n = 140) was observed among HD (median [IQR] of 10 [5–19] days) compared to SD (median [IQR] of 13 [6–22] days), differences were not significant (p = 0.31). We further analyzed whether other causes different from ARDS could explain the effect over mortality, but the distribution of pulmonary embolism and bacterial infection (with or without sepsis) was similar between both groups.
Among 396 patients treated at least 3 days before outcome, HD corticosteroids were associated with an increased risk of need for MV or death (adjusted OR 2.35, 95% CI 1.42–3.90, p = 0.001) compared to SD patients. No significant differences between both groups were observed in the risk of developing a severe ARDS (Table 2). We performed sensitivity analyses excluding patients hospitalized for no longer than 7 days or those treated with less than 5 days of corticosteroids and all results remained practically unchanged.
As age is a strong prognostic factor, we analyzed the effect of this variable in primary and secondary outcomes. Figure 2 shows the impact of HD of corticosteroids on mortality depending on the age of patients. The risk of death significantly increased in patients older than 65 years when compared to SD patients, being this effect attenuated in younger individuals. Age and HD of corticosteroids showed a trend towards a significant interaction between both (p = 0.076). The interaction between age and HD in the risk of need for VM or death was not significant (Fig. 1 of Supplemental Data).
Discussion
In this large observational study performed in Madrid, Spain, short-term high doses of corticosteroids, when compared with standard doses, were associated with an increased mortality and a higher need for MV or death in hospitalized patients with a SARS-CoV-2 infection developing an ARDS. This harmful effect was mainly observed in elderly patients, since young age attenuated the deleterious impact of higher doses.
Despite its known immunosuppressive effect, corticosteroids have been a treatment option for several bacterial, viral, or even fungal infections, especially in the most severe, in order to regulate excessive immune responses causing tissue damage [12]. Steroids have been extensively used in severe cases of SARS-CoV-2 infection since the beginning of the pandemics [13]. The rationale was based on preliminary data suggesting that the immune response has two distinct phases [14]: the first triggered by the virus itself and characterized by mild constitutional and respiratory symptoms, and the second consisting in an excessive inflammatory response leading to lung tissue injury and multisystem organ dysfunction in a minority of patients [15]. This hypothesis has been later supported by postmortem case series, as histopathology findings reinforce the role of immune-mediated, rather than pathogen-mediated, pulmonary inflammation, and death [16, 17]. In this setting, therapeutic immunomodulation in severe COVID-19 was initially proposed on the basis of a strong scientific rationale [3,4,5,6, 18]. Several drugs aimed to limit immune-mediated injury in COVID-19 are consequently being investigated, including corticosteroids [19,20,21,22]. This treatment option is not new for coronavirus infections, as they were already used during the corresponding outbreaks of SARS and Middle East Respiratory Syndrome (MERS). However, results for SARS were either inconclusive or even harmful for patients [23] and no impact on mortality was observed for MERS. In fact, coronavirus RNA clearance was delayed [24]. Among hospitalized COVID-19 patients, steroids have been the anti-inflammatory treatment most evaluated [13]. Small cohort studies and case series yielded mixed results, reporting both positive [2, 25,26,27] and negative [28,29,30] outcomes. However, these works are prone to imbalances, indication bias, and reverse causality. For example, a deleterious effect of steroids on influenza A–related critical illness was assumed until both baseline and time-dependent factors were considered in a later study [31]. For this reason, clinical trials are needed to provide the highest evidence for efficacy and safety. Preliminary results from a randomized, controlled, open-label trial comparing dexamethasone 6 mg given once daily for up to 10 days versus usual care in hospitalized COVID-19 patients in the UK showed that patients who were allocated to receive dexamethasone had a reduced rate of mortality compared to those who concurrently allocated standard of care. This benefit was observed only in patients receiving invasive MV and those who required supplemental oxygen, but not in patients with mild infection [7]. Additional clinical trials about the effect of different types and dosages of corticosteroids compared to placebo or standard care have been studied afterwards. Dexamethasone at a higher dose (up to 20 mg per day) for critically ill patients was also associated with a positive effect, measured as a composite of days alive and free of MV [32]. Conversely, neither hydrocortisone (up to 200 mg/day) [33, 34] nor methylprednisolone (0.5 mg/kg/day) [35] showed a significant difference in their respective primary endpoints compared to placebo or standard care. It is worth to mention the heterogeneity of the study population in the clinical trial with methylprednisolone (ranging from moderate to critically ill COVID-19 patients) [35]. On the other hand, given at a higher dose (40 mg bid), methylprednisolone for severe COVID-19 might be associated with a better evolution in a recent clinical trial, but results have to be interpreted with caution as sample size is small and participants were partially randomized, resulting in differences within baseline characteristics between both groups [36]. In a meta-analysis including all except two of the previous clinical trials, corticosteroids were associated with a lower risk of mortality after 28 days, with consistent results across most of the subgroups but with no evidence that a higher dose of steroids would result in a greater benefit than a lower dose [37].
For patients with an ARDS from any cause different from SARS-CoV-2 infection, general recommendations include the administration of corticosteroids at methylprednisolone equivalent of 1 mg/kg/day exclusively when a moderate or severe stage is observed [9]. Despite results from this initial clinical trial, there is uncertainty about whether higher doses of steroids in COVID-19 could provide a greater benefit, especially in the presence of a severe inflammation. To the best of our knowledge, no study has evaluated the effect of high dose (HD) pulse therapy compared to supportive care alone or standard doses (SDs). Our results outline that HDs are not associated with better outcomes, but with a higher risk of death and need for MV or death in comparison with SD. Although some differences in baseline characteristics were detected (male gender, older age, and comorbidities were significantly more common among the HD group), appropriate adjustments were performed, concomitant treatments during admission were equally administered, and sensitivity analyses reinforced the robustness of our results. In addition, despite all patients had a severe COVID-19, overall mortality (24.8%) was consistent with that reported from previous studies in the early stages of the pandemics in Europe among hospitalized subjects [38, 39], suggesting a lower risk of selection bias.
These results emphasize that, even if some immunomodulatory or immunosuppressive treatments might be effective for severe COVID-19, the role of adaptive immunity is essential as viral dissemination is a key driver of severe disease. The optimal aim in severe COVID-19 might be to achieve a complicate balance between controlling the excessive innate immune hyperactivation and recovering from the adaptive immune dysfunction, which has a critical role on clearing the viral infection and downregulating the innate immunity [3]. In fact, this may be the reason for the higher mortality observed in patients with moderate or severe immunosuppression when compared with general population [40,41,42,43], as well as the better outcomes whenever immunosuppression is non-severe [44]. A similar rationale may be applied to corticosteroids, aiming to modulate the immune hyperactivation without exerting a suppression of the adaptive immunity. In addition, higher doses could probably be associated with more and more serious adverse events, including concomitant infections [45], and clinical benefit should therefore exceed the increase of risks. Thus, standard doses given for the shorter time possible might be a better option for severe COVID-19 patients.
There are obvious limitations in a single-center study of this type. First, due to the observational character of the current work, potential confounding factors might have not been controlled, and, therefore, conclusions must be taken with caution. In addition, the lack of randomization could have introduced indication bias, using HD of corticosteroids for more severe patients. Second, the range of doses used in the HD group contributed to some heterogeneity within this group. And finally, standardized care pathways and evidence-based treatment protocols for COVID-19 have not been established, and management might have been different between patients, introducing potential bias.
Despite these limitations, these results could have direct relevance to the evolving management of COVID-19 for treating physicians. In addition to the contribution made by the increasing evidence of corticosteroids at moderate doses for severe COVID-19 [7, 32,33,34,35,36,37], we add robust evidence to prevent from using high doses of corticosteroids for these patients in order to avoid harmful effects.
In conclusion, among hospitalized patients with COVID-19 developing an ARDS, the administration of high doses of corticosteroids are associated with increased mortality and a higher risk of need for MV or death compared to standard doses. Thus, corticosteroids at moderate doses given for a short period might be more beneficial for these patients. Nevertheless, randomized, double-blind, controlled clinical trials are needed to provide stronger evidence in this regard.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization. Novel coronavirus – China. Available at: www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed 09 Jul 2020
Wu C, Chen X, Cai Y et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:1–11. https://doi.org/10.1001/jamainternmed.2020.0994
Vardhana SA, Wolchok JD (2020) The many faces of the anti-COVID immune response. J Exp Med 217:e20200678. https://doi.org/10.1084/jem.20200678
Mehta P, McAuley DF, Brown M et al (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 395:1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Zhang W, Zhao Y, Zhang F et al (2020) The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 214:108393. https://doi.org/10.1016/j.clim.2020.108393
Fu Y, Cheng Y, Wu Y (2020) Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin 35:266–271. https://doi.org/10.1007/s12250-020-00207-4
Randomised Evaluation of COVID-19 Therapy (RECOVERY). Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. 2020. Available at: https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed Jul 12, 2020
Beigel JH, Tomashek KM, Dodd LE, et al (2020) Remdesivir for the treatment of Covid-19–preliminary report. N Engl J Med :NEJMoa2007764. https://doi.org/10.1056/NEJMoa2007764
Annane D, Pastores SM, Rochwerg B et al (2017) Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med 45:2078–2088. https://doi.org/10.1097/CCM.0000000000002737
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD et al (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533. https://doi.org/10.1001/jama.2012.5669
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
McGee S, Hirschmann J (2008) Use of corticosteroids in treating infectious diseases. Arch Intern Med 168:1034–1046. https://doi.org/10.1001/archinte.168.10.1034
Tobaiqy M, Qashqary M, Al-Dahery S et al (2020) Therapeutic management of patients with COVID-19: a systematic review. Infection Prevention in Practice 2:100061. https://doi.org/10.1016/j.infpip.2020.100061
Siddiqi HK, Mehra MR (2020) COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 39:405–407. https://doi.org/10.1016/j.healun.2020.03.012
D’Antiga L (2020) Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 26:832–834. https://doi.org/10.1002/lt.25756
Carsana L, Sonzogni A, Nasr A et al (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis S1473–3099(20):30434–30435. https://doi.org/10.1016/S1473-3099(20)30434-5
Dorward DA, Russell CD, Um IH, et al (2020) Tissue-specific tolerance in fatal Covid-19. medRxiv. 10.1101/2020.07.02.20145003 [cited 2020 July 25]
Ye Q, Wang B, Mao J (2020) The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Inf Secur 80:607–613. https://doi.org/10.1016/j.jinf.2020.03.037
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (covid-19): a review. JAMA 323(18):1824–1836. https://doi.org/10.1001/jama.2020.6019
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20:363–374. https://doi.org/10.1038/s41577-020-0311-8
Scavone C, Brusco S, Bertini M, et al (2020) Current pharmacological treatments for COVID-19: what’s next?. Br J Pharmacol. https://doi.org/10.1111/bph.15072
Alzghari SK, Acuña VS (2020) Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol 127:104380. https://doi.org/10.1016/j.jcv.2020.104380
Stockman LJ, Bellamy R, Garner P (2006) SARS: systematic review of treatment effects. PLoS Med 3:e343. https://doi.org/10.1371/journal.pmed.0030343
Arabi YM, Mandourah Y, Al-Hameed F et al (2018) Corticosteroid therapy for critically ill patients with the Middle East respiratory syndrome. Am J Respir Crit Care Med 197:757–767. https://doi.org/10.1164/rccm.201706-1172OC
Kolilekas L, Loverdos K, Giannakaki S, et al (2020) Can steroids reverse the severe COVID-19 induced “cytokine storm”? J Med Virol. https://doi.org/10.1002/jmv.26165
Fadel R, Morrison AR, Vahia A et al (2020) Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020:ciaa601. https://doi.org/10.1093/cid/ciaa601
So C, Ro S, Murakami M, Imai R, Jinta T (2020) High-dose, short-term corticosteroids for ARDS caused by COVID-19: a case series. Respirol Case Rep 8:e00596. https://doi.org/10.1002/rcr2.596
Yuan M, Xu X, Xia D, Tao Z, Yin W, Tan W, Hu Y, Song C (2020) Effects of corticosteroid treatment for non-severe COVID-19 pneumonia: a propensity score-based analysis. Shock 54(5):638–643. https://doi.org/10.1097/SHK.0000000000001574
Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J (2020) The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Inf Secur 81:e13–e20. https://doi.org/10.1016/j.jinf.2020.03.062
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395:473–475. https://doi.org/10.1016/S0140-6736(20)30317-2
Delaney JW, Pinto R, Long J et al (2016) The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care 20:75. https://doi.org/10.1186/s13054-016-1230-8
Tomazini BM, Maia IS, Cavalcanti AB et al (2020) Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 324:1–11. https://doi.org/10.1001/jama.2020.17021
Dequin PF, Heming N, Meziani F et al (2020) Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 324:1–9. https://doi.org/10.1001/jama.2020.16761
Writing Committee for the REMAP-CAP Investigators, Angus DC, Derde L, Al-Beidh F et al (2020) Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 Corticosteroid Domain randomized clinical trial. JAMA 324:1317–1329. https://doi.org/10.1001/jama.2020.17022
Jeronimo CMP, Farias MEL, Val FFA et al (2020) Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 12:ciaa1177. https://doi.org/10.1093/cid/ciaa1177
Corral L, Bahamonde A, Arnaiz delas Revillas F et al (2020) GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. medRxiv 06(17):20133579. https://doi.org/10.1101/2020.06.17.20133579
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT), Working Group, JAC S, Murthy S, Diaz JV et al (2020) Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324:1–13. https://doi.org/10.1001/jama.2020.17023
Giacomelli A, Ridolfo AL, Milazzo L et al (2020) 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res 158:104931. https://doi.org/10.1016/j.phrs.2020.104931
Rodilla E, Saura A, Jiménez I et al (2020) Association of Hypertension with all-cause mortality among hospitalized patients with COVID-19. J Clin Med 9:E3136. https://doi.org/10.3390/jcm9103136
He W, Chen L, Chen L et al (2020) COVID-19 in persons with haematological cancers. Leukemia 34:1637–1645. https://doi.org/10.1038/s41375-020-0836-7
Martín-Moro F, Marquet J, Piris M et al (2020) Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol 190:e16–e20. https://doi.org/10.1111/bjh.16801
Pereira MR, Mohan S, Cohen DJ et al (2020) COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 20:1800–1808. https://doi.org/10.1111/ajt.15941
Akalin E, Azzi Y, Bartash R et al (2020) Covid-19 and kidney transplantation. N Engl J Med 382:2475–2477. https://doi.org/10.1056/NEJMc2011117
Monreal E, de la Maza S, Gullón P, et al (2020) Non-severe immunosuppression might be associated with a lower risk of moderate-severe acute respiratory distress syndrome in COVID-19. Research Square. https://doi.org/10.21203/rs.3.rs-27095/v1. [cited July 2020 25]
Stuck AE, Minder CE, Frey FJ (1989) Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 11:954–963. https://doi.org/10.1093/clinids/11.6.954
Acknowledgments
We thank all treating physicians belonging to the COVID-HRC group for their significant labor during the pandemics of COVID-19 in our center: The COVID-HRC (COronaVIrus Disease 2019—Hospital Ramón y Cajal) Group: Masjuan, J; Fortún, J; Montero-Errasquín, B; Manzano, L; Máiz-Carro, L; Sánchez-García, EM; Hidalgo, F; Domínguez, AR; Pérez-Molina, JA; Sánchez-Sánchez, O; Comeche, B; Monge-Maillo, B; Barbero, E; Barbolla-Díaz, I; Aranzábal Orgaz, L; Cobo, J; Rayo, I; Fernández-Golfín, C; González, E; Rincón-Díaz, LM; Ron, R; Mateos-Muñoz, B; Navas, E; Moreno, J; Norman, J; Serrano, S; Quereda Rodríguez-Navarro, C; Vallés, A; Herrera, S; Mateos del Nozal, J; Moreno-Cobo, MA; Gioia, F; Concejo-Badorrey, MC; Ortiz Barraza, EY; Moreno, A; Chamorro, S; Casado, JL; Almonacid, C; Nieto, R; Diz, S; Moreno, E; Conde, M; Hermida, JM; López, M; Monreal, E; Sainz de la Maza, S; Costa-Frossard, L; Natera-Villalba, E; Chico-García, JL; Beltrán-Corbellini, Á; Rodríguez-Jorge, F; Fernández-Velasco, JI; Rodríguez de Santiago, E; Rita, CG; Iturrieta-Zuazo, I; De Andrés, A; Espiño, M; Vázquez, M; Fernández Lucas, M; Martínez-Sanz, J; García-Barragán, N; Buisán, J; Toledano, R; Alonso-Canovas, A; Pérez-Torre, P; Matute-Lozano, MC; López-Sendón, JL; García-Ribas, G; Corral, Í; Villar, LM.
Author information
Authors and Affiliations
Consortia
Contributions
Drs Monreal, Sainz de la Maza, and Villar had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Monreal, Sainz de la Maza, Muriel, Zamora, Villar
Acquisition, analysis, or interpretation of data: Monreal, Sainz de la Maza, Natera-Villalba, Beltrán-Corbellini, Rodríguez-Jorge, Fernández-Velasco, Walo-Delgado, Alonso-Canovas, Fortún, Manzano, Montero-Errasquín, Costa-Frossard, Masjuan, Villar
Drafting of the manuscript: Monreal, Sainz de la Maza, Natera-Villalba, Villar
Critical revision of the manuscript for important intellectual content: Monreal, Sainz de la Maza, Natera-Villalba, Beltrán-Corbellini, Rodríguez-Jorge, Fernández-Velasco, Walo-Delgado, Muriel, Zamora, Alonso-Canovas, Fortún, Manzano, Montero-Errasquín, Costa-Frossard, Masjuan, Villar
Statistical analyses: Monreal, Muriel, Zamora
Administrative, technical, or material support: Monreal, Sainz de la Maza, Natera-Villalba, Beltrán-Corbellini, Rodríguez-Jorge, Fernández-Velasco, Walo-Delgado, Alonso-Canovas, Villar
Study supervision: Villar
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the institutional ethics board of Hospital Universitario Ramón y Cajal.
Consent to participate
Due to the nature of the retrospective chart review, the need for informed consent from individual patients was waived.
Consent for publication
All authors accept the terms and conditions of the editorial for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Monreal, E., Sainz de la Maza, S., Natera-Villalba, E. et al. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 40, 761–769 (2021). https://doi.org/10.1007/s10096-020-04078-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-04078-1