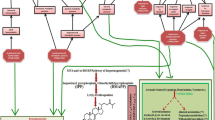
Abstract
Picrorhiza kurrooa synthesizes a large array of pharmacologically important monoterpenoid iridoid glycosides called picrosides. Although chemical profile and pharmacological activities of P. kurrooa have been extensively studied, limited attempts have been made to decipher the biosynthetic route and to identify the key regulatory genes involved in picroside biosynthesis. In the present study, NADPH–cytochrome P450 reductase, a key enzyme involved in electron transfer to cytochrome P450s was identified from P. kurrooa. The full length cDNA (2679 bp) contained an open reading frame of 2133 bp, corresponding to 710 amino acids. PkCPR was heterologously expressed in Escherichia coli and the kinetic parameters of the recombinant enzyme were determined. Specific activity, V max and K m of PkCPR were found to be 5.8 ± 0.05 μmol min−1 mg−1, 8.1 ± 0.12 μmol min−1 mg−1 and 7.8 μM, respectively. PkCPR was found to be spatially regulated at transcript level, being maximally expressed in leaf tissues. Altitude was found to have a positive effect on the picroside concentration and the picroside content positively correlated with the PkCPR transcript levels in samples collected at varied altitudes. Further, transcript profiling under methyl jasmonate, salicylic acid, 2,4-dicholorophenoxy acetic acid and UV-B elicitations displayed differential transcriptional regulation of PkCPR that fully corroborated with the identified cis-elements within the PkCPR promoter. Expression of PkCPR was inducible by UV-B and phytohormone elicitation, indicating that the PkCPR is possibly related to defence reactions, including biosynthesis of secondary metabolites. Present study is so far the only report of identification and functional characterization of CPR ortholog from P. kurrooa.










Similar content being viewed by others
Abbreviations
- IPTG:
-
Isopropyl-β-d-thiogalactopyranoside
- ORF:
-
Open reading frame
- CPR:
-
Cytochrome P450 reductase
- RACE:
-
Rapid amplification of cDNA ends
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- MeJA:
-
Methyl jasmonate
- SA:
-
Salicylic acid
- 2,4-D:
-
2,4-Dicholorophenoxy acetic acid
- SDS-PAGE:
-
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TSS:
-
Transcriptional start site
- UTR:
-
Untranslated region
References
Ansari RA, Tripathi SC, Patnaik GK, Dhawan BN (1991) Antihepatotoxic properties of picroliv: an active fraction from rhizomes of Picrorhiza kurrooa. J Ethnopharmacol 34(1):61–68
Bahuguna R, Purohit M, Rawat M, Purohit A (2000) Qualitative and quantitative variations in alkaloids of Aconitum species from Garhwal Himalaya. J Plant Biol 27(2):179–183
Barnes PW, Flint SD, Caldwell MM (1987) Photosynthesis damage and protective pigments in plants from a latitudinal arctic/alpine gradient exposed to supplemental UV-B radiation in the field. Arct Alp Res 19:21–27
Bartwal A, Mall R, Lohani P, Guru SK, Arora S (2013) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul 32(1):216–232. doi:10.1007/s00344-012-9272-x
Benveniste I, Gabriac B, Durst F (1986) Purification and characterization of the NADPH–cytochrome P-450 (cytochrome c) reductase from higher-plant microsomal fraction. Biochem J 235:365–373
Bhandari P, Kumar N, Singh B, Gupta AP, Kaul VK, Ahuja PS (2009) Stability-indicating LC–PDA method for determination of picrosides in hepatoprotective Indian herbal preparations of Picrorhiza kurroa. Chromatographia 69(3–4):221–227
Bhat WW, Dhar RS, Vishwakarma RA, Lattoo SK (2012a) Metabolic profiling of Picrorhiza kurrooa and molecular characterization of key regulatory genes of picroside biosynthesis. In: The 4th EMBO meeting, Nice, France. European Molecular biology Organisation, p 183
Bhat WW, Lattoo SK, Rana S, Razdan S, Dhar N, Dhar RS, Vishwakarma RA (2012b) Efficient plant regeneration via direct organogenesis and Agrobacterium tumefaciens-mediated genetic transformation of Picrorhiza kurroa: an endangered medicinal herb of the alpine Himalayas. In Vitro Cell Dev Plant 48:1–9
Bhat WW, Lattoo SK, Razdan S, Dhar N, Rana S, Dhar RS, Khan S, Vishwakarma RA (2012c) Molecular cloning, bacterial expression and promoter analysis of squalene synthase from Withania somnifera (L.) Dunal. Gene 499(1):25–36. doi:10.1016/j.gene.2012.03.004
Bhat WW, Dhar N, Razdan S, Rana S, Mehra R, Nargotra A, Dhar RS, Ashraf N, Vishwakarma R, Lattoo SK (2013) Molecular characterization of UGT94F2 and UGT86C4, two glycosyltransferases from Picrorhiza kurrooa: comparative structural insight and evaluation of substrate recognition. PLoS One. doi:10.1371/journal.pone.0073804
Brosché M, Fant C, Bergkvist SW, Strid H, Svensk A, Olsson O, Strid AÅ (1999) Molecular markers for UV-B stress in plants: alteration of the expression of four classes of genes in Pisum sativum and the formation of high molecular mass RNA adducts. Biochim Biophys Acta Gene Struct Expr 1447(2–3):185–198. doi:10.1016/S0167-4781(99)00154-2
Caldwell MM, Robberecht R, Flint SD (1983) Internal filters: prospects for UV acclimation in higher plants. Physiol Plant 58(3):445–450
Camas N, Radusiene J, Ivanauskas L, Jakstas V, Cirak C (2013) Altitudinal changes in the content of bioactive substances in Hypericum orientale and Hypericum pallens. Acta Physiol Plant :1–12. doi: 10.1007/s11738-013-1446-z
Chander R, Kapoor NK, Dhawan BN (1992) Picroliv, picroside-I and kutkoside from Picrorhiza kurrooa are scavengers of superoxide anions. Biochem Pharmacol 44(1):180–183
Clarke SM, Mur LA, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38(3):432–447
Dorsch W, Stuppner H, Wagner H, Gropp M, Demoulin S, Ring J (1991) Antiasthmatic effects of Picrorhiza kurroa: androsin prevents allergen- and PAF-induced bronchial obstruction in guinea pigs. Int Arch Allergy Appl Immunol 95(2–3):128–133
Duan H, Schuler MA (2006) Heterologous expression and strategies for encapsulation of membrane-localized plant P450s. Phytochem Rev 5(2–3):507–523
Eberle D, Ullmann P, Werck-Reichhart D, Petersen M (2009) cDNA cloning and functional characterisation of CYP98A14 and NADPH:cytochrome P450 reductase from Coleus blumei involved in rosmarinic acid biosynthesis. Plant Mol Biol 69(3):239–253. doi:10.1007/s11103-008-9420-7
El-Beltagi HS, Ahmed OK, El-Desouky W (2011) Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat Phys Chem 80(9):968–976. doi:10.1016/j.radphyschem.2011.05.002
Gahlan P, Singh HR, Shankar R, Sharma N, Kumari A, Chawla V, Ahuja PS, Kumar S (2012) De novo sequencing and characterization of Picrorhiza kurrooa transcriptome at two temperatures showed major transcriptome adjustments. BMC Genomics 13(1):126. doi:10.1186/1471-2164-13-126
Gupta A, Khajuria A, Singh J, Bedi KL, Satti NK, Dutt P, Suri KA, Suri OP, Qazi GN (2006) Immunomodulatory activity of biopolymeric fraction RLJ-NE-205 from Picrorhiza kurroa. Int Immunopharmacol 6(10):1543–1549. doi:10.1016/j.intimp.2006.05.002
Huang F-C, Sung P-H, Do Y-Y, Huang P-L (2012) Differential expression and functional characterization of the NADPH cytochrome P450 reductase genes from Nothapodytes foetida. Plant Sci 190:16–23
Jennewein S, Park H, DeJong JHM, Long RM, Bollon AP, Croteau RB (2005) Coexpression in yeast of Taxus cytochrome P450 reductase with cytochrome P450 oxygenases involved in Taxol biosynthesis. Biotechnol Bioeng 89(5):588–598
Joy K, Kuttan R (1999) Anti-diabetic activity of Picrorrhiza kurroa extract. J Ethnopharmacol 67(2):143–148
Joy K, Rajeshkumar N, Kuttan G, Kuttan R (2000) Effect of Picrorrhiza kurroa extract on transplanted tumours and chemical carcinogenesis in mice. J Ethnopharmacol 71(1):261–266
Katoch M, Fazli I, Suri K, Ahuja A, Qazi G (2011) Effect of altitude on picroside content in core collections of Picrorhiza kurrooa from the north western Himalayas. J Nat Med 65(3–4):578–582
Kawoosa T, Singh H, Kumar A, Sharma S, Devi K, Dutt S, Vats S, Sharma M, Ahuja P, Kumar S (2010) Light and temperature regulated terpene biosynthesis: hepatoprotective monoterpene picroside accumulation in Picrorhiza kurrooa. Funct Integr Genom 10(3):393–404. doi:10.1007/s10142-009-0152-9
Kawoosa T, Gahlan P, Devi AS, Kumar S (2013) The GATA and SORLIP motifs in the 3-hydroxy-3-methylglutaryl-CoA reductase promoter of Picrorhiza kurrooa for the control of light-mediated expression. Funct Integr Genom. doi:10.1007/s10142-013-0350-3
Keasling JD (2012) Synthetic biology and the development of tools for metabolic engineering. Metab Eng 14(3):189–195. doi:10.1016/j.ymben.2012.01.004
Koopmann E, Hahlbrock K (1997) Differentially regulated NADPH:cytochrome P450 oxidoreductases in parsley. Proc Natl Acad Sci U S A 94(26):14954–14959
Kumar V, Sood H, Sharma M, Chauhan RS (2013) A proposed biosynthetic pathway of picrosides linked through the detection of biochemical intermediates in the endangered medicinal herb Picrorhiza kurroa. Phytochem Anal 24(6):598–602
Madronich S, McKenzie RL, Björn LO, Caldwell MM (1998) Changes in biologically active ultraviolet radiation reaching the Earth's surface. J Photochem Photobiol B Biol 46(1):5–19
Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129(3):1003–1018. doi:10.1104/pp. 011001
Matsuura H, Costa F, Yendo A, Fett-Neto A (2013) Photoelicitation of bioactive secondary metabolites by ultraviolet radiation: Mechanisms, strategies, and applications. In: Chandra S, Lata H, Varma A (eds) Biotechnology for medicinal plants. Springer, Berlin, pp 171–190. doi:10.1007/978-3-642-29974-2_7
Meijer AH, Cardoso M, Voskuilen JT, Waal A, Verpoorte R, Hoge JHC (1993) Isolation and characterization of a cDNA clone from Catharanthus roseus encoding NADPH: cytochrome P-450 reductase, an enzyme essential for reactions catalysed by cytochrome P-450 mono-oxygenases in plants. Plant J 4(1):47–60
Mizutani M, Ohta D (1998) Two isoforms of NADPH:cytochrome P450 reductase in Arabidopsis thaliana. Gene structure, heterologous expression in insect cells, and differential regulation. Plant Physiol 116(1):357–367
Mondal TK, Bantawa P, Sarkar B, Ghosh P, Chand PK (2012) Cellular differentiation, regeneration, and secondary metabolite production in medicinal Picrorhiza spp. Plant Cell Tissue Organ 112:1–16
Morant M, Jorgensen K, Schaller H, Pinot F, Moller BL, Werck-Reichhart D, Bak S (2007) CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 19(5):1473–1487
Ohta D, Mizutani M (2004) Redundancy or flexibility: molecular diversity of the electron transfer components for P450 monooxygenases in higher plants. Front Biosci 9:1587–1597
Pandit S, Shitiz K, Sood H, Chauhan RS (2012a) Differential biosynthesis and accumulation of picrosides in an endangered medicinal herb Picrorhiza kurroa. J Plant Biochem Biotechnol 22(3):335–342
Pandit S, Shitiz K, Sood H, Naik PK, Chauhan RS (2012b) Expression pattern of fifteen genes of non-mevalonate (MEP) and mevalonate (MVA) pathways in different tissues of endangered medicinal herb Picrorhiza kurroa with respect to picrosides content. Mol Biol Rep 40(2):1053–1063. doi:10.1007/s11033-012-2147-1
Porter TD, Beck TW, Kasper CB (1990) NADPH–cytochrome P-450 oxidoreductase gene organization correlates with structural domains of the protein. Biochemistry 29(42):9814–9818
Rajkumar V, Guha G, Kumar RA (2011) Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. Food Chem Toxicol 49(2):363–369. doi:10.1016/j.fct.2010.11.009
Rana S, Lattoo SK, Dhar N, Razdan S, Bhat WW, Dhar RS, Vishwakarma R (2013) NADPH–cytochrome P450 reductase: molecular cloning and functional characterization of two paralogs from Withania somnifera (L.) Dunal. PLoS One 8(2):e57068
Reichhart D, Salaün J-P, Benveniste I, Durst F (1980) Time course of induction of cytochrome P-450, NADPH–cytochrome c reductase, and cinnamic acid hydroxylase by phenobarbital, ethanol, herbicides, and manganese in higher plant microsomes. Plant Physiol 66(4):600–604
Ro DK, Ehlting J, Douglas CJ (2002) Cloning, functional expression, and subcellular localization of multiple NADPH–cytochrome P450 reductases from hybrid poplar. Plant Physiol 130(4):1837–1851. doi:10.1104/pp. 008011
Saraswat B, Visen PK, Patnaik GK, Dhawan BN (1997) Protective effect of picroliv, active constituent of Picrorhiza kurrooa, against oxytetracycline induced hepatic damage. Indian J Exp Biol 35(12):1302–1305
Schreiner M, Mewis I, Huyskens-Keil S, Jansen MAK, Zrenner R, Winkler JB, O’Brien N, Krumbein A (2012) UV-B-induced secondary plant metabolites — potential benefits for plant and human health. Crit Rev Plant Sci 31(3):229–240. doi:10.1080/07352689.2012.664979
Schuler MA, Werck-Reichhart D (2003) Functional genomics of P450s. Annu Rev Plant Biol 54(1):629–667
Shet MS, Sathasivan K, Arlotto MA, Mehdy MC, Estabrook RW (1993) Purification, characterization, and cDNA cloning of an NADPH–cytochrome P450 reductase from mung bean. Proc Natl Acad Sci U S A 90(7):2890–2894
Singh H, Gahlan P, Dutt S, Ahuja PS, Kumar S (2011) Why uproot Picrorhiza kurrooa, an endangered medicinal herb? Curr Sci 100(7):1055–1059
Singh H, Gahlan P, Kumar S (2013) Cloning and expression analysis of ten genes associated with picrosides biosynthesis in Picrorhiza kurrooa. Gene 515(2):320–328
Sood H, Chauhan R (2010) Biosynthesis and accumulation of a medicinal compound, Picroside-I, in cultures of Picrorhiza kurroa Royle ex Benth. Plant Cell Tissue Organ 100(1):113–117
Spitaler R, Schlorhaufer PD, Ellmerer EP, Merfort I, Bortenschlager S, Stuppner H, Zidorn C (2006) Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. ARBO. Phytochemistry 67:409–417
Spitaler R, Winkler A, Lins I, Yanar S, Stuppner H, Zidorn C (2008) Altitudinal variation of phenolic contents in flowering heads of Arnica montana cv. ARBO: a 3-year comparison. J Chem Ecol 34(3):369–375. doi:10.1007/s10886-007-9407-x
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tripathy MK, Tyagi W, Goswami M, Kaul T, Singla-Pareek SL, Deswal R, Reddy MK, Sopory SK (2012) Characterization and functional validation of tobacco PLC Delta for abiotic stress tolerance. Plant Mol Biol Report 30(2):488–497
Turunen M, Latola K (2005) UV-B radiation and acclimation in timberline plants. Environ Pollut 137(3):390–403
Vermilion J, Ballou D, Massey V, Coon M (1981) Separate roles for FMN and FAD in catalysis by liver microsomal NADPH–cytochrome P-450 reductase. J Biol Chem 256(1):266–277
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5(5):283–291
Wang M, Roberts DL, Paschke R, Shea TM, Masters BSS, Kim J-JP (1997) Three-dimensional structure of NADPH–cytochrome P450 reductase: prototype for FMN-and FAD-containing enzymes. Proc Natl Acad Sci U S A 94(16):8411–8416
Yang C-Q, Lu S, Mao Y-B, Wang L-J, Chen X-Y (2010) Characterization of two NADPH: cytochrome P450 reductases from cotton (Gossypium hirsutum). Phytochemistry 71(1):27–35
Zhang L, Lu X, Shen Q, Chen Y, Wang T, Zhang F, Wu S, Jiang W, Liu P, Zhang L (2012) Identification of putative Artemisia annua ABCG transporter unigenes related to artemisinin yield following expression analysis in different plant tissues and in response to methyl jasmonate and abscisic acid treatments. Plant Mol Biol Rep 30(4):838–847
Acknowledgments
This work was supported by a grant from the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, under Network Project BSC0108. The authors are thankful to Dr. R.K. Khajuria, Instrumentation Division, Indian Institute of Integrative Medicine Jammu, India, for facilitating HPLC analysis. W.W. Bhat, S. Razdan, S. Rana and N. Dhar are highly thankful to Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, for Senior Research Fellowship (CSIR-SRF). S. A. Pandith is grateful to University Grants Commission, Government of India, New Delhi, for Senior Research Fellowship (UGC-SRF). This manuscript represents institutional communication number IIIM/1606/2013.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Nucleotide and the deduced amino acid sequence of PkCPR. 5′ UTR and 3′ UTR are shown in italics. The dash marks the translation termination codon (PPTX 1401 kb)
Fig. S2
Transmembrane domain prediction of PkCPR using Phobious (a) and TMHMM (b) web server (PPTX 79 kb)
Fig. S3
Phylogenetic tree of PkCPR and other plant CPR genes constructed using the ClustalW2 program and MEGA 5.2 software based on the neighbour-joining method. A total of 15 protein sequences used for analysis were from following plant species: Picrorhiza kurrooa (AEW43314), Perilla frutescens (ADC94831), Salvia miltiorrhiza (AGL46979), Catharanthus roseus (Q05001), Petroselinum crispum (AAB97737; AAB97736), Withania somnifera (ADI49691; ADG29353), Pisum sativum (AAC09468), Arabidopsis thaliana (CAA46814; NP_194750), Gossypium hirsutum (ACN54323; ACN54324) and Petunia × hybrida (AAZ39648; AAZ39649) (PPTX 71 kb)
Fig. S4
HPLC chromatogram of standard (picroside I and picroside II mix) at 270 nm (a) and standard (apocynin, androsin and feruloylcatalpol) at 283 nm (b). HPLC chromatogram of the Dhanwas sample of Picrorhiza kurrooa showing five marker compounds viz. picroside I, picroside II, apocynin, androsin and feruloylcatalpol (c and d) (PPTX 387 kb)
Fig. S5
Nucleotide sequences of the PkCPR gene promoter. Numbering starts from the predicted transcription start site. The putative core promoter consensus sequences and the motifs with significant similarity to the previously identified cis-acting elements are boxed and the names are given (PPTX 629 kb)
Fig. S6
Ramachandran plot of PkCPR 3D model of Picrorhiza using PROCHECK server. Most favoured regions are coloured red (A, B, L), additional allowed (a, b, l, p), generously allowed (~a, ~b, ~l, ~p), and disallowed regions are indicated as yellow, light yellow and white fields, respectively (PPTX 2437 kb)
Rights and permissions
About this article
Cite this article
Bhat, W.W., Rana, S., Dhar, N. et al. An inducible NADPH–cytochrome P450 reductase from Picrorhiza kurrooa — an imperative redox partner of cytochrome P450 enzymes. Funct Integr Genomics 14, 381–399 (2014). https://doi.org/10.1007/s10142-014-0362-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-014-0362-7