Abstract
Floral signaling, especially through floral scent, is often highly complex, and little is known about the molecular mechanisms and evolutionary causes of this complexity. In this study, we focused on the evolution of “floral scent genes” and the associated changes in their functions in three closely related orchid species of the genus Gymnadenia. We developed a benchmark repertoire of 2,571 expressed sequence tags (ESTs) in Gymnadenia odoratissima. For the functional characterization and evolutionary analysis, we focused on eugenol synthase, as eugenol is a widespread and important scent compound. We obtained complete coding complementary DNAs (cDNAs) of two copies of putative eugenol synthase genes in each of the three species. The proteins encoded by these cDNAs were characterized by expression and testing for activity in Escherichia coli. While G. odoratissima and Gymnadenia conopsea enzymes were found to catalyze the formation of eugenol only, the Gymnadenia densiflora proteins synthesize eugenol, as well as a smaller amount of isoeugenol. Finally, we showed that the eugenol and isoeugenol producing gene copies of G. densiflora are evolutionarily derived from the ancestral genes of the other species producing only eugenol. The evolutionary switch from production of one to two compounds evolved under relaxed purifying selection. In conclusion, our study shows the molecular bases of eugenol and isoeugenol production and suggests that an evolutionary transition in a single gene can lead to an increased complexity in floral scent emitted by plants.
Similar content being viewed by others
Introduction
Many insect-pollinated plants communicate with their pollinators through a number of floral signals, such as fragrance, unique flower shape, and color (Delle-Vedove et al. 2011; Huber et al. 2005; Salzmann et al. 2007; Salzmann and Schiestl 2007; Schiestl and Glaser 2012; Streinzer et al. 2010; van der Niet et al. 2011a, b; Vereecken and Schiestl 2009). Floral scents are among the key signals in many plant-pollinator systems for attracting pollinators from both short and long distances (Brodmann et al. 2008; Huber et al. 2005; Majetic et al. 2007; Miyake and Yafuso 2003; Negre et al. 2003; Plepys et al. 2002) and play a vital role in the reproductive success of night-flowering plants, wherein visual cues are usually of minor importance for mediating interactions with pollinators (Balao et al. 2011; Dötterl and Jürgens 2005; Gupta et al. 2012; Kondo et al. 2006; Wälti et al. 2009). In addition, several studies have established that floral volatiles can serve multiple functions, such as attracting pollinators and deterring the nectar thieves simultaneously (Junker and Blüthgen 2010; Shuttleworth and Johnson 2009; Theis 2006; Theis et al. 2009). In plant signaling, volatile blends derived from vegetative tissues are generally assumed to enhance predator-mediated defenses, whereas floral-based chemicals are strictly considered insect attractants (Heil 2008; Pichersky and Gershenzon 2002; Raguso 2008; van Dam 2009). Increasing evidence, however, suggests that floral-specific chemical substances can also play a central role in guarding the reproductive organs from the antagonists (Kessler and Baldwin 2007; Kessler et al. 2013; Schiestl et al. 2011).
Gymnadenia odoratissima, Gymnadenia conopsea, and Gymnadenia densiflora rarely hybridize despite being sympatrically distributed (Lönn et al. 2006; Schiestl and Schlüter 2009; Soliva and Widmer 1999). Therefore, besides postzygotic isolation barriers, floral scent has been argued to be one of the most crucial factors to maintain reproductive isolation among these species (Huber et al. 2005; Schiestl and Schlüter 2009). Although three Gymnadenia species emit quantitatively and qualitatively different blends of approximately 50 volatile compounds to attract different suits of pollinators, eugenol and benzyl acetate are the only two shared active compounds among the scent bouquets of these species (Huber et al. 2005; Schiestl et al. 2011). However, in addition to these active compounds, other compounds found in blend can also influence plant performance. For example, Schiestl et al. (2011) uncovered that the key active compounds (e.g., eugenol and phenylacetaldehyde) emitted by G. odoratissima are positively associated with fitness. However, “non-active” compounds, such as (Z)-isoeugenol, limonene, and α-pinene are negatively correlated with fitness, possibly by interaction with blends of active volatile compounds that altogether can reduce pollinator visitations or increase florivores attractiveness.
In orchids, most of the previous molecular studies have predominantly made progress either in identifying MADS-box genes for flower development (Aceto and Gaudio 2011; Mondragón-Palomino and Theißen 2009; Mondragón-Palomino and Theißen 2011; Mondragón-Palomino and Theissen 2008; Xu et al. 2006) or in molecular phylogeny (Bellusci et al. 2008; Douzery et al. 1999; Inda et al. 2012; Mant et al. 2002; Soliva et al. 2001; van der Niet et al. 2011b). Our current knowledge of biosynthetic pathways that produce scent compounds in orchids, however, is still in its infancy. The food rewarding Gymnadenia species emits a large amount of different scent compounds, and therefore, it provides an excellent model system to understand the evolutionary processes underlying striking scent variability and to study the genes responsible for scent biosynthesis. Surprisingly, to date, nothing is known about the scent biosynthesis in this species.
Despite the increasing number of expressed sequence tags (ESTs) and genomic resources for several orchid species in public databases, sequence information for G. odoratissima had remained elusive until the present study (Chan et al. 2011; Fu et al. 2012; Hsiao et al. 2006, 2011; Monteiro et al. 2012; Pan et al. 2012; Sedeek et al. 2013; Teh et al. 2011). Therefore, to characterize genes involved in floral- and scent-related pathways, we have constructed one standard and one subtracted complementary DNA (cDNA) library and developed a benchmark EST resource of 2,571 sequences from G. odoratissima. The floral scent compounds, eugenol and isoeugenol, which constitute a group of phenylpropene compounds, are structurally distinguished from each other only by the double-bond position of the propene side chain (Koeduka et al. 2006, 2008). These compounds have also received historical attention due to their flavoring and antimicrobial properties (Ahmed et al. 2000; Koeduka et al. 2006; Moleyar and Narasimham 1992; Sangwan et al. 1990). Enzymes responsible for the synthesis of eugenol, isoeugenol, and related compounds have been functionally characterized in model plants, such as Petunia (Koeduka et al. 2008), Clarkia (Koeduka et al. 2006, 2008), Ocimum (Koeduka et al. 2006), and Anise (Koeduka et al. 2009). All of these studies confer that eugenol and isoeugenol are catalyzed by two different types of enzyme (Koeduka et al. 2006, 2008). However, there is still a lack of comprehensive knowledge about evolution of such scent producing genes in plants. In this study, with the use of functional genomics and evolutionary approaches, we investigate a G. odoratissima EST resource as a starting point and further address about eugenol and isoeugenol emission in naturally occurring three Gymnadenia species, and also about the identification, expression, and evolution of the genes underlying their synthesis.
Methods
Plant materials
G. odoratissima, G. conopsea, and G. densiflora flowers and leaves were harvested from natural populations of Ofenpass (G. odoratissima), Albulapass (G. conopsea), and Münstertal (G. densiflora) in Switzerland. These samples were immediately frozen in liquid nitrogen and stored in a −80 °C freezer until subsequent analyses. Total RNA was isolated from these floral and leaf materials using RNeasy mini kit (Qiagen AG, Hombrechtikon, Switzerland). The necessary permits for all collection locations were obtained from the Amt für Natur und Umwelt, Chur, Switzerland, for the described field studies.
Standard cDNA library construction
Approximately 1–2 mg of poly(A)+ RNA from G. odoratissima flowers was obtained using Oligotex mRNA isolation kit following the manufacturer’s instructions (Qiagen AG, Hombrechtikon, Switzerland). The Creator smart cDNA library construction kit (Clontech, Palo Alto, CA, USA) was employed for the construction of one standard cDNA library, and larger than 1 kb inserts were selected using Zymoclean™ Gel DNA Recovery Kit (Zymo Research, Orange, CA, USA) instead of using the size selected columns provided with cDNA library kit (Clontech, Palo Alto, CA, USA). Prepared oligo(dT)-primed inserts were spliced into pDNR-LIB vector (Clontech, Palo Alto, CA, USA). Subsequently, the ligation mixture was transformed in to XL-10 gold Kan+ ultra-competent cells of Escherichia coli (Stratagene, La Jolla, CA, USA). White colonies were screened to ensure the efficiency of prepared library and insert size range through colony PCR reactions. The library was preserved for long-term storage at −80 °C with the addition of 20 % (v/v) of glycerol in a final concentration and delivered to Purdue University sequencing platform, West Lafayette, IN, USA, for sequencing.
Suppressive subtractive hybridization cDNA library construction
G. odoratissima first-strand cDNA was prepared from 2 μg of poly(A)+ floral RNA (tester population) and leaf (driver population) following Smart PCR cDNA synthesis kit specifications (Clontech, Palo Alto, CA, USA). The subtraction was based on Clontech kit (Clontech, Palo Alto, CA, USA) and was performed as described in the PCR-Select cDNA subtraction kit user manual. Subsequently to this step, resulting cDNA pool was used for size selection (>1 kb) as described above. The gel purified cDNA was end-repaired using DNA terminator kit (Lucigen Corporation, Middleton, WI, USA), and blunt end-polished cDNA fragments were cloned into pSMART HC kan vector (Lucigen Corporation, Middleton, WI, USA). The resulting ligation mixture was transformed into XL-10 gold ultracompetent cells (Stratagene, La Jolla, CA, USA). One suppressive subtractive hybridization (SSH) cDNA library (flower vs leaf) was constructed and delivered for sequencing as described above.
Bioinformatic tools
In this study, one standard (flower specific) and one SSH (flower vs leaf) cDNA libraries were constructed from G. odoratissima. Altogether, 2,571 ESTs from the total pool of 3,456 cDNA clones were achieved with a high confidence base call. Among these high-quality ESTs, 1,376 ESTs were generated from SSH library, whereas 1,195 ESTs were obtained from standard library. The chromatograms retrieved from sequencing platform were analyzed using the Phred base-calling program with default parameters (Ewing et al. 1998). Sequences were subjected to remove vector sequences with the use of Cross-match program. Masked sequences were filtered out from the pool of total sequences. We analyzed these sequences with their corresponding quality files produced from Phred analysis for generating a tentatively unique gene (TUG) set from each library. These sequences were used for Phrap assembly program using default parameters. Clustering step estimated sequence redundancy in libraries. BlastN searches against “other EST database” and protein similarities (BlastX) against non-redundant (nr) protein database (Altschul et al. 1990) were analyzed with an E-value cutoff of ≤e−10 and ≤e−6, respectively.
Assignment of gene ontology terms
Unigenes from both the libraries were annotated with gene ontology terms using Blast2GO software (Conesa et al. 2005). These unigenes were annotated using BlastX search against GenBank nr database. The best 20 hits were evaluated for assigning the gene ontology (GO) terms with an E-value cutoff of ≤e−6.
Volatile collection for quantitative eugenol and isoeugenol GC analysis
A total of 14 to 20 G. odoratissima (Ofenpass), G. conopsea (Albulapass), and G. densiflora (Münstertal) individuals were chosen for scent collections. All volatile collections were sampled during the day time (between 11 AM and 5 PM) between July and August 2008 in the field. Each undamaged whole inflorescence was separately covered with an oven roasting bag (Toppits®, Cafresco frischhalteprodukte GmbH & Co., Minden, Germany), and these bags were subsequently closed with a plastic wire at both ends. A battery-operated pump (PAS-500 Personal Air Sampler, Spectrex, Redwood city, California, USA) was employed for each setup to absorb the volatile compounds into glass tubes prepared with 100 mg of Tenax TA (Gerstel GmbH & Co., Mülheim an der Ruhr, Germany). Each volatile collection was carried out for 30 min. In addition to Gymnadenia volatile collections, control samples were also collected in order to determine the possible air contaminants in the surrounding environment. After sealing both ends of each glass tube with Teflon tape, each sample was kept in a Gerstel TDS storage tube (Gerstel GmbH & Co., Mülheim an der Ruhr, Germany) and stored at −20 °C.
Collected scent samples were processed within 5 days using a gas chromatograph (GC, Agilent 6890 N) fitted with both a HP5 column (30-m × 0.32-mm internal diameter × 0.25-μm film thickness) and an Agilent 5975 mass selective detector connected to a thermal desorption system (TDS2, Gerstel, Mühlheim, Germany) for scent analysis. The TDS temperature was programmed to rise from 30 °C (0.5-min hold) to 240 °C (1-min hold) at 60 °C/min. The cold injection system (CIS) temperature was programmed to rise from −50 °C (0.5-min hold) to 150 °C (0.5-min hold) at 16 °C/s and to 250 °C (0.5-min hold) at 12 °C/s. The oven temperature of the GC6890 was adjusted to rise from 50 °C (3-min hold) to 230 °C at 8 °C/min. Agilent ChemStation and MSD ChemStation E.02.00.493 (Agilent Technologies, Palo Alto, CA, USA) software were not only used for identification of peak but also for retention time. Synthetic eugenol (Sigma-Aldrich, Switzerland) and isoeugenol (Sigma-Aldrich, Switzerland) standards were analyzed to compare the retention time and for calibration of the peak area. Quantification of eugenol and isoeugenol amounts were performed in terms of nanograms per liter of air sampled. To statistically compare amounts of eugenol and isoeugenol emitted by plants of the three species, we used a one-way ANOVA with LSD post hoc tests. Correlations between eugenol and isoeugenol were assessed by Pearson product moment correlations (SPSS Inc., Chicago).
Isolation of full-length Gymnadenia eugenol cDNAs and preparation of constructs
Several partial sequences showing high similarities with eugenol synthase genes from standard and subtracted EST libraries were initially screened for the isolation of full-length G. odoratissima eugenol synthase genes. The largest sequence among these partial sequences was further used to design gene-specific forward and reverse primers. Two full-length cDNA sequences encoding G. odoratissima eugenol synthase1 (GoEGS1) and G. odoratissima eugenol synthase2 (GoEGS2) were isolated using SMART-RACE cDNA amplification kit (Clontech). Amplified cDNA fragments were ligated into the pSMART HC kan vector (Lucigen Corporation, Middleton, WI, USA). Cloned sequences were verified using the ABI Prism Big Dye terminator cycle sequencing kit on ABI automated sequencers (Applied Biosystems) following the manufacturer’s instructions. The complete coding regions were obtained for G. conopsea eugenol synthase1 (GcEGS1), G. conopsea eugenol synthase2 (GcEGS2), G. densiflora (iso)eugenol synthase1 (GdIEGS1), and G. densiflora (iso)eugenol synthase2 (GdIEGS2) based on the GoEGS1 and GoEGS2 sequences. RT-PCR reactions were set up for amplifying coding regions (ORFs) of GoEGS1, GoEGS2, GcEGS1, GcEGS2, GdIEGS1, and GdIEGS2 from flower cDNA using forward and reverse primers but without stop codons in the end of sequences (Supplementary Table 1). The PCR conditions were as follows: 2 min initial denaturation at 94 °C, 30 cycles of 1 min at 94 °C, 1 min at 55 °C and 2 min at 72 °C. The final primer extension cycle was extended to 9 min. The amplified Gymnadenia coding regions were spliced into the expression vector using pEXP-CT/Topo TA expression kit according to the manufacturer’s instructions. The orientation of all prepared constructs was confirmed by complete sequencing.
Biochemical test for activity in E. coli
The expression vectors with the cDNAs were transferred to E. coli cells, and the cells were grown as previously described (Koeduka et al. 2006). After induction and the addition of coniferyl alcohol, cells were grown overnight. Thereafter, the medium was collected and assayed for the presence of eugenol and isoeugenol (Koeduka et al. 2006).
Semi-quantitative RT-PCR analyses
To test gene expression patterns between flower and leaf tissue, two individuals each from natural populations of G. odoratissima (Ofenpass), G. conopsea (Davos), and G. densiflora (Münstertal) were selected. Total RNA followed by mRNA isolation from these individuals was performed as described above. About 1 μg of poly(A)+ RNA was reverse-transcribed using Omniscript RT kit (Qiagen AG, Hombrechtikon, Switzerland). The cDNA amplification was performed in a Biometra thermocycler (Biometra GmbH, Germany) following these PCR conditions: 2 min initial denaturation at 94 °C, 38 cycles of 1 min at 94 °C, 1 min at 55 °C, and 2 min at 72 °C, followed by final primer extension for 5 min at 72 °C. Amplification of the cDNA coding for the putative G. odoratissima glyceraldehyde-3-phosphate dehydrogenase (GADPH), a ubiquitously expressed gene, served as a control for cDNA synthesis and PCR efficiency in all samples (Supplementary Table 1). Seven microliters of the amplified PCR products from each sample was electrophoresed on 1.5 % TAE agarose gels and stained with ethidium bromide.
Phylogeny reconstruction and selection analysis
GoEGS2 gene sequence was exploited for searching its homologous sequences from NCBI using BlastN algorithm (nr/nt database) with the “somewhat similar sequences” option (Max target sequences 100, expect threshold 10, world size 11, and match/mismatch scores 2–3). These sequences were added to the pool of six Gymnadenia eugenol gene sequences and other functionally characterized eugenol or isoeugenol synthase genes. The sequence alignments were manually performed by employing BioEdit v7.0.5, and poorly aligned sequences were not taken into account for subsequent analyses. Mr. Modeltest 2.3 (Nylander 2004) and MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) were applied for determining the best substitution model and approximating the posterior probabilities of the phylogenetic tree by employing Markov chain Monte Carlo (MCMC) method, respectively. BaseML, a part of PAML 4.4 package (Yang 2007), was implemented for optimizing the branch lengths of resultant consensus tree. In addition, CodeML was further applied for a codon-based model for calculating the synonymous and non-synonymous substitution rates.
Results
G. odoratissima EST library overview
Of the 2,571 high-quality G. odoratissima EST sequences, 1,195 and 1,376 EST sequences were retained after vector clipping, adaptor removal, and trimming of poor-quality sequence from the standard and subtraction cDNA libraries, respectively. Table 1 illustrates the summary statistics for the produced EST sequences from each library. Altogether, the success rate for obtaining sequences of high-quality sequence data was 76.4 %. The average sequence length for both libraries was 541 bp with the proportion of observed redundancy of 33 % (standard library) and 66 % (subtraction library).
Unigene blast searches with the publically available database
Our BLASTX results suggest that 12.09 % (standard) and 17.37 % (subtraction) of the unigenes represent no significant similarities with other plant sequences, indicating that these sequences may have a specific role in Gymnadenia and other closely related plant species. Furthermore, BlastN similarity searches against EST others db yielded 61.97 and 59.11 % unigenes similarities from standard and subtracted EST libraries, respectively. Supplementary Tables S2 and S3 summarize the most abundant ESTs from standard and subtraction EST collections, and these transcripts possess at least eleven or more overlapping sequences in each contig with its putative identification determined by BLASTX analysis.
Gene ontology annotations for standard and subtraction libraries
Of the total 802 Gymnadenia standard library unigenes, 556 unigenes (69.33 %) were defined by biological process terms, 518 (64.59 %) were defined by cellular component terms, and 572 (71.32 %) were defined by molecular function terms using Blast2GO. Relative frequencies from both libraries were determined and compared using GO terms (Supplementary Fig. S1). In total, 802 unigene sequences of standard library were annotated in 4,500 GO terms. We also estimated that a total of 266 unigenes from standard library were having enzyme code, whereas for subtraction library, only 144 unigenes were having enzyme code. To examine relative differential expression of transcripts between both libraries, we also compared the gene ontology annotations between standard and subtraction libraries (Supplementary Fig. S1). A total of 291 (61.65 %), 255 (54.03 %), and 239 (50.64 %) unigenes from subtraction EST libraries were defined by molecular function, biological process, and cellular component, respectively. Altogether, 472 unigenes from substraction library were annotated in 2,113 GO terms.
Emission of eugenol and isoeugenol in Gymnadenia species
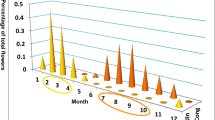
In the three investigated species, eugenol was produced by G. odoratissima and G. densiflora, but only in trace amounts in a few individuals of G. conopsea (F 2,46 = 23.02, P < 0.001; Table 2). Isoeugenol was found predominantly in G. densiflora and only in trace amounts in some individuals of the other two species (F 2,46 = 11.14, P < 0.001; Table 2). There was a strong positive correlation between eugenol and isoeugenol in G. densiflora (r = 0.75, P = 0.001).
Identification of Gymnadenia cDNAs encoding enzyme capable of synthesizing eugenol and isoeugenol
Two complete coding cDNA sequences of 984-bp long were identified and named as GoEGS1 and GoEGS2. Both nucleotide sequences encoded a peptide sequence of 328 aa with a calculated molecular mass of 36.2 kDa and 6.13 pI (isoelectric point). The deduced proteins of GoEGS1 and GoEGS2 showed 55 and 54 % amino acid identity with Clarkia EGS2 and Petunia EGS1, respectively (Supplementary Fig. S2). A total of four complete coding regions each with 984-bp long were isolated from G. conopsea and G. densiflora species and designated as GcEGS1, GcEGS2, GdIEGS1, and GdIEGS2 with calculated molecular masses of 36.25, 36.21, 36.33, and 36.33 kDa, respectively. All these sequences were nearly identical, varying by 1 to 12 amino acids between same species. Since it has been established that E. coli cells growing on coniferyl alcohol will take up this compound and convert it to coniferyl acetate, the substrate of both eugenol and isoeugenol synthases (Koeduka et al. 2006), we tested the activity of the Gymnadenia enzymes by expressing them in E. coli cells. These cells expressing GoEGS1, GoEGS2, GcEGS1, and GcEGS2 and growing in the presence of coniferyl alcohol produced only eugenol, whereas the E. coli cells expressing GdIEGS1 and GdIEGS2 produced eugenol, as well as a trace amount of isoeugenol (Figs. 1 and 2; Supplementary Fig. S3 and Supplementary Table S4).
Gene expression in Gymnadenia floral and leaf tissue
All the EGS and IEGS genes are expressed in floral tissues that are the key source for fragrance emission in Gymnadenia species (Stpiczynska 2001). None of the Gymnadenia EGS showed any expression in leaf tissue, indicating that all functionally characterized EGS and IEGS in this study are florally expressed (Supplementary Fig. S4).
Evolutionary analysis
Altogether, 56 sequences were used for evolutionary analysis. All the functionally characterized Gymnadenia eugenol gene sequences form one distinct clade together with two monocot sequences (Supplementary Fig. S5). Furthermore, our phylogenetic analysis is also consistent with the previous study showing that CbIGS1, CbEGS1, PhIGS1, PaAIS1, and ObEGS1 are closely related and clustered into a single clade (Koeduka et al. 2009). Our results further determine that CbEGS2 and PhEGS1 are only distantly related to all other functionally characterized eugenol and isoeugenol producing genes and belong to completely separate clade. Our selection analysis revealed significant relaxed purifying selection (ω = 0.48; P = 0.024) acting only on the GdIEGS1 and GdIEGS2 clade, while no other functionally characterized GoEGS1, GoEGS2, GcEGS1, and GcEGS2 genes showed evidence for selection (Supplementary Fig. S5, Supplementary Tables S5 and S6). Furthermore, our evolutionary analyses also indicate that Petunia EGS1 is under purifying selection.
Discussion
In this study, we identified the genes encoding eugenol and (iso)eugenol synthase in the orchid genus Gymnadenia. We show that a single enzyme, (iso)eugenol synthase in G. densiflora catalyses eugenol together with trace amounts of isoeugenol and this double-function is evolutionarily derived from the single-product enzyme. Although it was previously known that single scent enzymes can produce several different scent compounds (Falara et al. 2011; Garms et al. 2010; Martin et al. 2004), we demonstrate here that the increased functional complexity evolved under relaxed purifying selection. The production of more than one compound by a single enzyme may contribute to the reproductive biology and/or defence system of the plant.
Besides other sequenced ESTs from orchid genera, such as Phalaenopsis, Oncidium, Ophrys, and Vanda using Sanger or next-generation sequencing approaches (Chan et al. 2011; Hsiao et al. 2006, 2011; Sedeek et al. 2013; Tan et al. 2005; Teh et al. 2011), our study reports a significant resource of 2,571 ESTs derived from floral tissue of Gymnadenia odoratissima (Orchidoideae, Orchidinae). Floral tissues were preferred for libraries construction to cover the large range of genes involved in flower senescence, flower pigmentation, and fragrance-related attributes. The primary objective of these EST generations was to identify the floral- and fragrance-related genes in our focal species. In the standard Gymnadenia library, transcripts that had third highest expression were found to be homologous to phenylacetaldehyde synthase gene. Fragrance-related transcripts further served as the basis for gene characterization in Gymnadenia species. Identified putative genes are the first representation of “scent genes” in Gymnadenia. On the other hand, a highly expressed transcript found in our subtraction library was cytochrome P450, which is not only entailed in primary/secondary metabolism, but probably also in promoting longer survival ability (Hsiao et al. 2011). Despite the small number of sequences generated for G. odoratissima, it was striking that EST sequences corresponded to all important categories of gene ontology terms. Altogether, it verifies that our small number of ESTs build up an important resource for future molecular research in orchids.
To date, NADP-dependent reductases that produce eugenol, isoeugenol, and t-anol have only been identified in eudicot species (Koeduka et al. 2006, 2008, 2009). Although some orchids produce copious amount of these compounds, no monocot NADP-dependent phenylpropene enzyme has been reported up to now (Huber et al. 2005; Johnson et al. 2007; Nishida et al. 2004; Schiestl et al. 2011). The four GoEGS1, GoEGS2, GcEGS1, and GcEGS2 are responsible for formation of eugenol, as evidenced by our functional characterization. Considering the high levels of sequence similarities among all the characterized Gymnadenia enzymes, it was expected that GdIEGS1 and GdIEGS2 will also catalyze the formation of eugenol only. However, these enzymes catalyze a mixture of eugenol and isoeugenol. It is common for terpene synthases to be able to form multiple products from a single prenyl diphosphate substrate (Chen et al. 2011). Whereas terpene synthases often show multiple products, as also recently shown in orchids, Vanda Mimi Palmer (Song et al. 2012) and Phalaenopsis bellina (Hsiao et al. 2008), previously characterized eugenol synthase and isoeugenol synthase made only one product. Koeduka et al. (2008) has shown that the amino acids at positions 84 and 87 in CbIGS1 and similar positions in other functionally characterized proteins, are crucial for specifying either eugenol or isoeugenol production.
We also show that the Gymnadenia enzymes share a high degree of protein identity with Clarkia and Petunia EGSs (Koeduka et al. 2008). Moreover, the two sequences from G. odoratissima, G. conopsea, and G. densiflora were nearly identical, and it is as yet not clear whether they represent two different alleles of the same locus or two duplicated gene copies. Interestingly, we found an evolutionary switch from the enzymatic production of one scent compound in G. odoratissima and G. conopsea, to the production of two compounds in G. densiflora. Since we also found significant values of selection for this evolutionary switch, we interpret this transition, leading to the production of an additional scent compound, to be mediated by relaxed purifying selection (ω = 0.48). At present, it is, however, unknown what could be the possible fitness impact of the additional volatile production. We suggest two hypotheses that may account for the evolution of isoeugenol emission in G. densiflora:
-
1.
Better discrimination by pollinators: Although G. conopsea and G. densiflora are very similar in their floral color and morphology, they may be incompatible due to different genome size and ploidy levels at least in some populations (Trávníček et al. 2011). Enhanced differences in floral scent may increase prezygotic isolation by pollinators and thus increase reproductive success in mixed populations. However, there is evidence for little prezygotic, pollinator-mediated isolation among the two Gymnadenia species (Jersáková et al. 2010).
-
2.
Better defense: Isoeugenol has been shown to be under negative directional selection mediated by pollinators in G. odoratissima, suggesting it can deter insects from flowers (Schiestl et al. 2011). Similarly, in Petunia, isoeugenol emission has recently been shown in reducing the floral damage from its florivores by 50 % (Kessler et al. 2013). Isoeugenol may thus play a role in reducing insect-mediated damage to flowers, and eventually benefit plant fitness.
References
Aceto S, Gaudio L (2011) The MADS and the beauty: genes involved in the development of orchid flowers. Curr Genomics 12:342–356
Ahmed M, Amin S, Islam M, Takahashi M, Okuyama E, Hossain CF (2000) Analgesic principle from Abutilon indicum. Pharmazie 55:314–316
Altschul S, Gish W, Miller W, Myers E, Lipman D (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Balao F, Herrera J, Talavera S, Dötterl S (2011) Spatial and temporal patterns of floral scent emission in Dianthus inoxianus and electroantennographic responses of its hawkmoth pollinator. Phytochemistry 72:601–609
Bellusci F, Pellegrino G, Palermo A, Musacchio A (2008) Phylogenetic relationships in the orchid genus Serapias L based on noncoding regions of the chloroplast genome. Mol Phylogenet Evol 47:986–991
Brodmann J, Twele R, Francke W, Holzler G, Zhang Q, Ayasse M (2008) Orchids mimic green-leaf volatiles to attract prey-hunting wasps for pollination. Curr Biol 18:740–744
Chan W-S, Abdullah JO, Namasivayam P (2011) Isolation, cloning, and characterization of fragrance-related transcripts from Vanda Mimi Palmer. Sci Hortic 127:388–397
Chen F, Tholl D, Bohlmann J, Pichersky E (2011) The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J 66:212–229
Conesa A, Gotz S, Garcia-Gomez J, Terol J, Talon M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676
Delle-Vedove R, Juillet N, Bessière J-M, Grison C, Barthes N, Pailler T, Dormont L, Schatz B (2011) Colour-scent associations in a tropical orchid: three colours but two odours. Phytochemistry 72:735–742
Dötterl S, Jürgens A (2005) Spatial fragrance patterns in flowers of Silene latifolia: lilac compounds as olfactory nectar guides? Plant Syst Evol 255:99–109
Douzery EJP, Pridgeon AM, Kores P, Linder HP, Kurzweil H, Chase MW (1999) Molecular phylogenetics of diseae (Orchidaceae): a contribution from nuclear ribosomal ITS sequences. Am J Bot 86:887–899
Ewing B, Hillier L, Wendl M, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185
Falara V, Akhtar TA, Nguyen TTH, Spyropoulou EA, Bleeker PM, Schauvinhold I, Matsuba Y, Bonini ME, Schilmiller AL, Last RL, Schuurink RC, Pichersky E (2011) The tomato terpene synthase gene family. Plant Physiol 157:770–789
Fu SF, Tsai T, Chen YR, Liu CP, Haiso LJ, Syue LH, Yeh HH, Huang HJ (2012) Characterization of the early response of the orchid, Phalaenopsis amabilis, to Erwinia chrysanthemi infection using expression profiling. Physiol Plant 145:406–425
Garms S, Köllner TG, Boland W (2010) A multiproduct terpene synthase from Medicago truncatula generates cadalane sesquiterpenes via two different mechanisms. J Org Chem 75:5590–5600
Gupta AK, Akhtar TA, Widmer A, Pichersky E, Schiestl FP (2012) Identification of white campion (Silene latifolia) guaiacol O-methyltransferase involved in the biosynthesis of veratrole, a key volatile for pollinator attraction. BMC Plant Biol 12:158
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61
Hsiao Y, Tsai W, Kuoh C, Huang T, Wang H, Wu T, Leu Y, Chen W, Chen H (2006) Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce the monoterpene biosynthesis pathway. BMC Plant Biol 6:14
Hsiao Y, Jeng M, Tsai W, Chung Y, Li C, Wu T, Kuoh C, Chen W, Chen H (2008) A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD(X)2-4D motif. Plant J 55:719–733
Hsiao Y-Y, Chen Y-W, Huang S-C, Pan Z-J, Fu C-H, Chen W-H, Tsai W-C, Chen H-H (2011) Gene discovery using next-generation pyrosequencing to develop ESTs for Phalaenopsis orchids. BMC Genomics 12:360
Huber F, Kaiser R, Sauter W, Schiestl F (2005) Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 142:564–575
Inda LA, Pimentel M, Chase MW (2012) Phylogenetics of tribe orchideae (Orchidaceae: Orchidoideae) based on combined DNA matrices: inferences regarding timing of diversification and evolution of pollination syndromes. Ann Bot 110:71–90
Jersáková J, Castro S, Sonk N, Milchreit K, Schödelbauerová I, Tolasch T, Dötterl S (2010) Absence of pollinator-mediated premating barriers in mixed-ploidy populations of Gymnadenia conopsea s.l. (Orchidaceae). Evol Ecol 24:1199–1218
Johnson SD, Ellis A, Dötterl S (2007) Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae). Am J Bot 94:47–55
Junker RR, Blüthgen N (2010) Floral scents repel facultative flower visitors, but attract obligate ones. Ann Bot 105:777–782
Kessler D, Baldwin IT (2007) Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J 49:840–854
Kessler D, Diezel C, Clark DG, Colquhoun TA, Baldwin IT (2013) Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecol Lett 16:299–306
Koeduka T, Fridman E, Gang DR, Vassão DG, Jackson BL, Kish CM, Orlova I, Spassova SM, Lewis NG, Noel JP, Baiga TJ, Dudareva N, Pichersky E (2006) Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc Natl Acad Sci 103:10128–10133
Koeduka T, Louie GV, Orlova I, Kish CM, Ibdah M, Wilkerson CG, Bowman ME, Baiga TJ, Noel JP, Dudareva N, Pichersky E (2008) The multiple phenylpropene synthases in both Clarkia breweri and Petunia hybrida represent two distinct protein lineages. Plant J 54:362–374
Koeduka T, Baiga TJ, Noel JP, Pichersky E (2009) Biosynthesis of t-anethole in Anise: characterization of t-anol/isoeugenol synthase and an O-methyltransferase specific for a C7-C8 propenyl side chain. Plant Physiol 149:384–394
Kondo M, Oyama-Okubo N, Ando T, Marchesi E, Nakayama M (2006) Floral scent diversity is differently expressed in emitted and endogenous eomponents in Petunia axillaris lines. Ann Bot 98:1253–1259
Lönn M, Alexandersson R, Gustafsson S (2006) Hybrids and fruit set in a mixed flowering-time population of Gymnadenia conopsea (Orchidaceae). Hereditas 143:222–228
Majetic C, Raguso R, Tonsor S, Ashman T-L (2007) Flower color-flower scent associations in polymorphic Hesperis matronalis (Brassicaceae). Phytochemistry 68:865–874
Mant J, Schiestl F, Peakall R, Weston P (2002) A phylogenetic study of pollinator conservatism among sexually deceptive orchids. Evolution 56:888–898
Martin DM, Fäldt J, Bohlmann J (2004) Functional characterization of nine norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol 135:1908–1927
Miyake T, Yafuso M (2003) Floral scents affect reproductive success in fly-pollinated Alocasia odora (Araceae). Am J Bot 90:370–376
Moleyar V, Narasimham P (1992) Antibacterial activity of essential oil components. Int J Food Microbiol 16:337–342
Mondragón-Palomino M, Theissen G (2008) MADS about the evolution of orchid flowers. TRENDS Plant Sci 13:51–59
Mondragón-Palomino M, Theißen G (2009) Why are orchid flowers so diverse? Reduction of evolutionary constraints by paralogues of class B floral homeotic genes. Ann Bot 104:583–594
Mondragón-Palomino M, Theißen G (2011) Conserved differential expression of paralogous DEFICIENS- and GLOBOSA-like MADS-box genes in the flowers of Orchidaceae: refining the ‘orchid code’. Plant J 66:1008–1019
Monteiro F, Sebastiana M, Figueiredo A, Sousa L, Cotrim H, Pais M (2012) Labellum transcriptome reveals alkene biosynthetic genes involved in orchid sexual deception and pollination-induced senescence. Funct Integr Genom 12:693–703
Negre F, Kish CM, Boatright J, Underwood B, Shibuya K, Wagner C, Clark DG, Dudareva N (2003) Regulation of methylbenzoate emission after pollination in Snapdragon and Petunia flowers. Plant Cell Online 15:2992–3006
Nishida R, Tan K-H, Wee S-L, Hee AK-W, Toong Y-C (2004) Phenylpropanoids in the fragrance of the fruit fly orchid, Bulbophyllum cheiri, and their relationship to the pollinator, Bactrocera papayae. Biochem Syst Ecol 32:245–252
Nylander J (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
Pan IC, Liao D-C, Wu F-H, Daniell H, Singh ND, Chang C, Shih M-C, Chan M-T, Lin C-S (2012) Complete chloroplast genome sequence of an orchid model plant candidate: Erycina pusilla apply in tropical Oncidium breeding. PLoS ONE 7:e34738
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Plepys D, Ibarra F, Löfstedt C (2002) Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae). Oikos 99:69–74
Raguso RA (2008) Wake up and smell the roses: the ecology and evolution of floral scent. Annu Rev Ecol Evol Syst 39:549–569
Ronquist F, Huelsenbeck J (2003) MrBayes 3:Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Salzmann CC, Schiestl FP (2007) Odour and colour polymorphism in the food-deceptive orchid Dactylorhiza romana. Plant Syst Evol 267:37–45
Salzmann C, Nardella A, Cozzolino S, Schiestl FP (2007) Variability in floral scent in rewarding and deceptive orchids: the signature of pollinator-imposed selection? Ann Bot 100:757–765
Sangwan NK, Verma BS, Verma KK, Dhindsa KS (1990) Nematicidal activity of some essential plant oils. Pestic Sci 28:331–335
Schiestl FP, Glaser F (2012) Specific ant-pollination in an alpine orchid and the role of floral scent in attracting pollinating ants. Alp Bot 122:1–9
Schiestl FP, Schlüter PM (2009) Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu Rev Entomol 54:425–446
Schiestl FP, Huber F, Gomez J (2011) Phenotypic selection on floral scent: trade-off between attraction and deterrence? Evol Ecol 25:237–248
Sedeek KE, Qi W, Schauer MA, Gupta AK, Poveda L, Xu S, Liu ZJ, Grossniklaus U, Schiestl FP, Schluter PM (2013) Transcriptome and proteome data reveal candidate genes for pollinator attraction in sexually deceptive orchids. PLoS ONE 8:e64621
Shuttleworth A, Johnson S (2009) Specialized pollination in the African milkweed Xysmalobium orbiculare: a key role for floral scent in the attraction of spider-hunting wasps. Plant Syst Evol 280:37–44
Soliva M, Widmer A (1999) Genetic and floral divergence among sympatric populations of Gymnadenia conopsea s.l. (Orchideaceae) with different flowering phenology. Int J Plant Sci 160:897–905
Soliva M, Kocyan A, Widmer A (2001) Molecular phylogenetics of the sexually deceptive orchid genus Ophrys (Orchidaceae) based on nuclear and chloroplast DNA sequences. Mol Phylogenet Evol 20:78–88
Song AAL, Abdullah JO, Abdullah MP, Shafee N, Rahim RA (2012) Functional expression of an orchid fragrance gene in Lactococcus lactis. Int J Mol Sci 13:1582–1597
Stpiczynska M (2001) Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae). Acta Soc Bot Pol 70:91–96
Streinzer M, Ellis T, Paulus H, Spaethe J (2010) Visual discrimination between two sexually deceptive Ophrys species by a bee pollinator. Arthropod Plant Interact 4:141–148
Tan J, Wang H, Yeh K (2005) Analysis of organ-specific, expressed genes in Oncidium orchid by subtractive expressed sequence tags library. Biotechnol Lett 27:1517–1528
Teh S, Chan W, Abdullah J, Namasivayam P (2011) Development of expressed sequence tag resources for Vanda Mimi Palmer and data mining for EST-SSR. Mol Biol Rep 38:3903–3909
Theis N (2006) Fragrance of Canada thistle (Cirsium arvense) Attracts both floral herbivores and pollinators. J Chem Ecol 32:917–927
Theis N, Kesler K, Adler LS (2009) Leaf herbivory increases floral fragrance in male but not female Cucurbita pepo subsp. texana (Cucurbitaceae) flowers. Am J Bot 96:897–903
Trávníček P, Kubátová B, Čurn V, Rauchová J, Krajníková E, Jersáková J, Suda J (2011) Remarkable coexistence of multiple cytotypes of the Gymnadenia conopsea aggregate (the fragrant orchid): evidence from flow cytometry. Ann Bot 107:77–87
van Dam NM (2009) Belowground herbivory and plant defenses. Annu Rev Ecol Evol Syst 40:373–391
van der Niet T, Hansen DM, Johnson SD (2011a) Carrion mimicry in a South African orchid: flowers attract a narrow subset of the fly assemblage on animal carcasses. Ann Bot 107:981–992
van der Niet T, Liltved W, Johnson S (2011b) More than meets the eye: a morphological and phylogenetic comparison of long-spurred, white-flowered Satyrium species (Orchidaceae) in South Africa. Bot J Linn Soc 166:417–430
Vereecken NJ, Schiestl FP (2009) On the roles of colour and scent in a specialized floral mimicry system. Ann Bot 104:1077–1084
Wälti M, Page P, Widmer A, Schiestl F (2009) How to be an attractive male: floral dimorphism and attractiveness to pollinators in a dioecious plant. BMC Evol Biol 9:190
Xu YF, Teo LL, Zhou J, Kumar PP, Yu H (2006) Floral organ identity genes in the orchid Dendrobium crumenatum. Plant J 46:54–68
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591
Acknowledgments
The authors appreciate Prof. Natalia Dudareva and Dr. Phillip SanMiguel for their help in sequencing cDNA libraries. Thanks are also due to Edward Connor for providing help in volatile collections and Philipp Schlüter for assisting in PAML program. We also thank the “Amt für Natur und Umwelt, Chur” for issuing collection permits. This work was financed by the Swiss National Science Foundation grant to FPS (SNF; project no. 31003A-112342) and ETH Zürich doctoral scholarship to AKG.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Primers used for Gymnadenia species (DOC 35 kb)
Table S2
List of most abundant transcripts from standard cDNA library and their gene annotations based on BlastX best hits (Cutoff ≤ e-6) (DOC 37 kb)
Table S3
List of most abundant transcripts from subtraction cDNA library and their gene annotations based on BlastX best hits (Cutoff ≤ e-6) (DOC 58 kb)
Table S4
Normalized instrument response to eugenol, isoeugenol, and indole (DOC 32 kb)
Table S5
Sequences used for evolutionary analysis (DOC 73 kb)
Table S6
Analysis of selection by employing PAML software (DOC 40 kb)
Figure S1
Gene Ontology classification showing relative differential expression of transcripts between Gymnadenia odoratissima standard (Std) and subtraction (SSH) EST libraries. (DOC 149 kb)
Figure S2
Alignment of deduced amino acid sequences of Gymnadenia and other functionally characterized NADPH-dependent family members. White letters on black background without asterisk represent amino acids similarities of at least seven sequences and additional asterisks below black backgrounds illustrate conserved amino acids in all sequences. The putative NADPH-binding domain is underlined in red. Species details and Genebank accession numbers of other characterized enzymes are shown in brackets: CbEGS1 (Clarkia breweri, EF467239.1), ObEGS1 (Ocimum basilicum, DQ372812.1), PhEGS1 (Petunia hybrida, EF467241.1), CbEGS2 (Clarkia breweri, EF467240.1), PhIGS1 (Petunia hybrida, DQ372813.1), CbIGS1 (Clarkia breweri, EF467238.1) and PaAIS1 (Pimpinella anisum, EU925388.1). (DOC 651 kb)
Figure S3
GC-MS analysis showing product formation by GoEGS1 and GoEGS2 in Gymnadenia odoratissima. (DOC 195 kb)
Figure S4
RT-PCR amplification showing eugenol and (iso) eugenol synthase gene expression using flower and leaf tissues. M, EGS, and IEGS reperesent size standard , eugenol and (iso) eugenol synthase genes, respecetively. Flower samples used as: 1, 2, 7, and 8 from G. odoratissima, 3, 4, 9, and 10 from G. conopsea, and 5, 6, 11, and 12 from G. densiflora and leaves tissue used as: 13, 14, 19, and 20 from G. odoratissima, 15, 16, 21, and 22 from G. conopsea and 17, 18, 23, and 24 from G. densiflora (DOC 117 kb)
Figure S5
Evolutionary analysis for Gymnadenia EGS homologs. The numbers represent posterior probabilities in phylogenetic tree. (DOC 165 kb)
Rights and permissions
About this article
Cite this article
Gupta, A.K., Schauvinhold, I., Pichersky, E. et al. Eugenol synthase genes in floral scent variation in Gymnadenia species. Funct Integr Genomics 14, 779–788 (2014). https://doi.org/10.1007/s10142-014-0397-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-014-0397-9