Abstract
We propose a multiscale mechanobiological model of bone remodelling to investigate the site-specific evolution of bone volume fraction across the midshaft of a femur. The model includes hormonal regulation and biochemical coupling of bone cell populations, the influence of the microstructure on bone turnover rate, and mechanical adaptation of the tissue. Both microscopic and tissue-scale stress/strain states of the tissue are calculated from macroscopic loads by a combination of beam theory and micromechanical homogenisation. This model is applied to simulate the spatio-temporal evolution of a human midshaft femur scan subjected to two deregulating circumstances: (i) osteoporosis and (ii) mechanical disuse. Both simulated deregulations led to endocortical bone loss, cortical wall thinning and expansion of the medullary cavity, in accordance with experimental findings. Our model suggests that these observations are attributable to a large extent to the influence of the microstructure on bone turnover rate. Mechanical adaptation is found to help preserve intracortical bone matrix near the periosteum. Moreover, it leads to non-uniform cortical wall thickness due to the asymmetry of macroscopic loads introduced by the bending moment. The effect of mechanical adaptation near the endosteum can be greatly affected by whether the mechanical stimulus includes stress concentration effects or not.










Similar content being viewed by others
Notes
The stress components \(\sigma ^\text {tissue}_{yy}\) and \(\sigma ^\text {tissue}_{zz}\) do not participate directly to the transfer of the resultant force \({\varvec{N}}\) and resultant bending moment \({\varvec{M}}\) across the bone cross section; however, they are accounted for in the calculation of the tissue-scale strain energy density \(\varPsi ^\text {tissue}\).
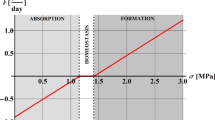
Parfitt reports that cortical bone of average bone volume fraction 0.95 has a turnover rate of 0.115 \(\hbox {cm}^3/\text {day}\), corresponding to \(\overline{\chi _\text {BV}}(0.95) \approx 0.77\,\times \,10^{-4}/\text {day}\) with \(\text {TV}^{\text {cort}} = 1.5\,\times \,10^{6}~\hbox {mm}^{3}\). He also reports that trabecular bone of average bone volume fraction 0.20 has a turnover rate of 0.25 \(\hbox {cm}^3/\text {day}\), corresponding to \(\overline{\chi _\text {BV}}(0.20) \approx 1.43\,\times \,10^{-4}/\text {day}\) with \(\text {TV}^{\text {trab}} = 1.75\,\times \,10^{6}\,\hbox {mm}^{3}\).
For the simulation in case (iii), the mechanical transduction strength parameters are: \(\kappa = 19\) pM/day and \(\lambda = 0.5\), calibrated with the tissue-scale mechanical stimulus while simulating spaceflight.
References
Adachi T, Aonuma Y, Ito SI, Tanaka M, Hojo M, Takano-Yamamoto T, Kamioka H (2009a) Osteocyte calcium signaling response to bone matrix deformation. J Biomech 42(15):2507–2512
Adachi T, Aonuma Y, Tanaka M, Hojo M, Takano-Yamamoto T, Kamioka H (2009) Calcium response in single osteocytes to locally applied mechanical stimulus: Differences in cell process and cell body. J Biomech 42(12):1989–1995. doi:10.1016/j.jbiomech.2009.04.034
Ashman R, Cowin S, Van Buskirk W, Rice J (1984) A continuous wave technique for the measurement of the elastic properties of cortical bone. J Biomech 17(5):349–361. doi:10.1016/0021-9290(84)90029-0
Ausk BJ, Huber P, Poliachik SL, Bain SD, Srinivasan S, Gross TS (2012) Cortical bone resorption following muscle paralysis is spatially heterogeneous. Bone 50(1):14–22
Ausk BJ, Huber P, Srinivasan S, Bain SD, Kwon RY, McNamara EA, Poliachik SL, Sybrowsky CL, Gross TS (2013) Metaphyseal and diaphyseal bone loss in the tibia following transient muscle paralysis are spatiotemporally distinct resorption events. Bone 57(2):413–422
Bauchau OA, Craig JI (2009) Structural analysis. With applications to aerospace structures, solid mech edn. Springer, Berlin
Bloomfield SA, Allen MR, Hogan HA, Delp MD (2002) Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone 31(1):149–157
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26(2):229–38
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42(4):606–15
Bousson V, Meunier A, Bergot C, Vicaut E, Rocha MA, Morais MH, Laval-Jeantet AM, Laredo JD (2001) Distribution of intracortical porosity in human midfemoral cortex by age and gender. J Bone Miner Res 16(7):1308–17. doi:10.1359/jbmr.2001.16.7.1308
Buenzli PR, Sims NA (2015) Quantifying the osteocyte network in the human skeleton. Bone (In Press), doi:10.1016/j.bone.2015.02.016
Buenzli PR, Pivonka P, Gardiner BS, Smith DW (2012) Modelling the anabolic response of bone using a cell population model. J Theor Biol 307:42–52
Buenzli PR, Thomas CDL, Clement JG, Pivonka P (2013) Endocortical bone loss in osteoporosis: the role of bone surface availability. Int J Numer Methods Biomed Eng 29(12):1307–1322. doi:10.1002/cnm.2567
Buenzli PR, Pivonka P, Smith DW (2014) Bone refilling in cortical basic multicellular units: insights into tetracycline double labelling from a computational model. Biomech Model Mechanobiol 13(1):185–203. doi:10.1007/s10237-013-0495-y
Burger EH, J Klein-Nulend, Jand Smit TH (2003) Strain-derived canalicular fluid flow regulates osteoclast activity in a remodelling osteon: a proposal. J Biomech 36:1453–1459. doi:10.1016/S0021-9290(03)00126-X
Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S (2010) A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: Relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res 25(12): 2558–2571
Burr D (1997) Muscle strength, bone mass, and age related bone loss. J Bone Miner Res 12(10):1547–1551. doi:10.1359/jbmr.1997.12.10.1547
Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM (2001) Intermittently administered human parathyroid hormone(1–34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res 16(1):157–165. doi:10.1359/jbmr.2001.16.1.157
Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, Vettorazzi E, Zustin J, Hahn M, Ager JW, Püschel K, Amling M, Ritchie RO (2013) Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci Transl Med 5(193):1–11. doi:10.1126/scitranslmed.3006286
Carpenter RD, Carter DR (2008) The mechanobiological effects of periosteal surface loads. Biomech Model Mechanobiol 7(3):227–242. doi:10.1007/s10237-007-0087-9
Carter DR, Beaupré GS (2001) Skeletal function and form. Cambridge University Press, Cambridge
Carter DR, Hayes WC (1977) The compressive behavior porous of bone structure as a two-phase. J Bone Joint Surg 59(7):954–962
Chappard D, Retailleau-Gaborit N, Legrand E, Baslé MF, Audran M (2005) Comparison insight bone measurements by histomorphometry and microCT. J Bone Miner Res 20(7):1177–1184
Christen P, Ito K, Müller R, Rubin MR, Dempster DW, Bilezikian JP, Van Rietbergen B (2012) Patient-specific bone modelling and remodelling simulation of hypoparathyroidism based on human iliac crest biopsies. J Biomech 45(14):2411–2416
Christen P, Ito K, Santos AAD, Müller R, van Rietbergen B (2013) Validation of a bone loading estimation algorithm for patient-specific bone remodelling simulations. J Biomech 46(5):941–948
Collet P, Uebelhart D, Vico L, Moro L, Hartmann D, Roth M, Alexandre C (1997) Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone 20(6):547–551
Cordey J, Gautier E (1999) Strain gauges used in the mechanical testing of bones. Part III: strain analysis, graphic determination of the neutral axis. Injury 30:SA21–SA25
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 28(1):2–17. doi:10.1002/jbmr.1805
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34:869–880
Eshelby JD (1957) The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proc R Soc A Math Phys Eng Sci 241(1226):376–396
Fan X, Roy E, Zhu L, Murphy TC, Ackert-Bicknell C, Hart CM, Rosen C, Nanes MS, Rubin J (2004) Nitric oxide regulates receptor activator of nuclear factor-kappaB ligand and osteoprotegerin expression in bone marrow stromal cells. Endocrinology 145(2):751–759. doi:10.1210/en.2003-0726
Feik SA, Thomas CD, Clement JG (1997) Age-related changes in cortical porosity of the midshaft of the human femur. J Anat 191:407–416
Feik SA, Thomas CD, Bruns R, Clement JG (2000) Regional variations in cortical modeling in the femoral mid-shaft: sex and age differences. Am J Phys Anthropol 112(2):191–205
Felsenberg D, Boonen S (2005) The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27(1):1–11
Forner-Cordero A, Koopman HJFM, Van der Helm FCT (2006) Inverse dynamics calculations during gait with restricted ground reaction force information from pressure insoles. Gait Posture 23(2):189–199
Fritsch A, Hellmich C, Dormieux L (2009) Ductile sliding between mineral crystals followed by rupture of collagen crosslinks: experimentally supported micromechanical explanation of bone strength. J Theor Biol 260(2):230–252. doi:10.1016/j.jtbi.2009.05.021
Fritsch A, Hellmich C (2007) ’Universal’ microstructural patterns in cortical and trabecular, extracellular and extravascular bone materials: micromechanics-based prediction of anisotropic elasticity. J Theor Biol 244(4):597–620. doi:10.1016/j.jtbi.2006.09.013
Frost HM (1987) Bone “mass” and the “mechanostat”: a proposal. Anat Rec 219(1):1–9. doi:10.1002/ar.1092190104
Frost HM (1997) On our age-related bone loss: insights from a new paradigm. J Bone Miner Res 12(10):1539–1546. doi:10.1359/jbmr.1997.12.10.1539
Frost HM (2003) Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol 275(2):1081–1101. doi:10.1002/ar.a.10119
Fyhrie DP, Carter DR (1986) A unifying principle relating stress to trabecular bone morphology. J Orthop Res 4(3):304–317
Fyhrie DP, Kimura JH (1999) Cancellous bone biomechanics. J Biomech 32(11):1139–1148. doi:10.1016/S0021-9290(99)00114-1
García-Aznar JM, Rueberg T, Doblare M (2005) A bone remodelling model coupling micro-damage growth and repair by 3D BMU-activity. Biomech Model Mechanobiol 4(2–3):147–167. doi:10.1007/s10237-005-0067-x
Gitman IM, Askes H, Kuhl E, Aifantis EC (2010) Stress concentrations in fractured compact bone simulated with a special class of anisotropic gradient elasticity. Int J Solids Struct 47(9):1099–1107. doi:10.1016/j.ijsolstr.2009.11.020
Hellmich C, Ulm FJ, Dormieux L (2004) Can the diverse elastic properties of trabecular and cortical bone be attributed to only a few tissue-independent phase properties and their interactions? Arguments from a multiscale approach. Biomech Model Mechanobiol 2(4):219–238. doi:10.1007/s10237-004-0040-0
Hellmich C, Kober C, Erdmann B (2008) Micromechanics-based conversion of CT data into anisotropic elasticity tensors, applied to FE simulations of a mandible. Ann Biomed Eng 36(1):108–122. doi:10.1007/s10439-007-9393-8
Hernandez CJ, Beaupré GS, Keller TS, Carter DR (2001) The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone 29(1):74–78
Hill R (1963) Elastic properties of reinforced solids. J Mech Phys Solids 11(5):357–372
Hill R (1965) A self-consistent mechanics of composite materials. J Mech Phys Solids 13(4):213–222. doi:10.1016/0022-5096(65)90010-4
Hipp JA, Edgerton BC, An KN, Hayes WC (1990) Structural consequences of transcortical holes in long bones loaded in torsion. J Biomech 23:1261–1268
Hirano T, Burr DB, Cain RL, Hock JM (2000) Changes in geometry and cortical porosity in adult, ovary-intact rabbits after 5 months treatment with LY333334 (hPTH 1–34). Calcif Tissue Int 66:456–460. doi:10.1007/s002230010091
Hjelmstad KD (2005) Fundamentals of structural mechanics, 2nd edn. Springer, Berlin
Huiskes R, Ruimerman R, Van Lenthe GH, Janssen JD (2000) Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405(6787):704–706. doi:10.1038/35015116
Jepsen KJ, Andarawis-Puri N (2012) The amount of periosteal apposition required to maintain bone strength during aging depends on adult bone morphology and tissue-modulus degradation rate. J Bone Miner Res 27(9):1916–1926. doi:10.1002/jbmr.1643
Jones SH (1974) Secretory territories and rates of matrix production of osteoblasts. Calcif Tissue Res 14:309–315
Judex S, Garman R, Squire M, Busa B, Donahue LR, Rubin C (2004) Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res 19(4):607–613. doi:10.1359/JBMR.040110
Kasiri S, Taylor D (2008) A critical distance study of stress concentrations in bone. J Biomech 41:603–609. doi:10.1016/j.jbiomech.2007.10.003
Kaspar D, Seidl W, Neidlinger-Wilke C, Beck A, Claes L, Ignatius A (2002) Proliferation of human-derived osteoblast-like cells depends on the cycle number and frequency of uniaxial strain. J Biomech 35(7):873–880. doi:10.1016/S0021-9290(02)00058-1
Kerschnitzki M, Kollmannsberger P, Burghammer M, Duda GN, Weinkamer R, Wagermaier W, Fratzl P (2013) Architecture of the osteocyte network correlates with bone material quality. J Bone Miner Res 28(8):1837–1845. doi:10.1002/jbmr.1927
Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I (2000) Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev 37: 225–233
Knothe Tate ML, Knothe U, Niederer P (1998) Experimental eludication of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. Am J Med Sci 316(3):189–195
Knothe Tate ML (2003) Whither flows the fluid in bone? An osteocyte’s perspective. J Biomech 36(10):1409–1424. doi:10.1016/S0021-9290(03)00123-4
Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A (2004) Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19(6):1006–1012
Lanyon LE, Goodship AE, Pye CJ, MacFie JH (1982) Mechanically adaptive bone remodelling. J Biomech 15(3):141–154
Lanyon LE, Rubin CT (1984) Static versus dynamic loads as an influence on bone remodelling. J Biomech 17(12):897–905
Laws N (1977) The determination of stress and strain concentrations at an ellipsoidal inclusion in an anisotropic material. J Elast 7(1):91–97
Leblanc AD, Spector ER, Evans HJ, Sibonga JD (2007) Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7(1):33–47
Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ (2004) Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J Theor Biol 229(3):293–309. doi:10.1016/j.jtbi.2004.03.023
Lerebours C, Thomas C, Clement J, Buenzli P, Pivonka P (2015) The relationship between porosity and specific surface in human cortical bone is subject specific. Bone 72:109–117. doi:10.1016/j.bone.2014.11.016
Levenston ME, Carter DR (1998) An energy dissipation-based model for damage stimulated bone adaptation. J Biomech 31(7):579–586
Lloyd DG, Besier TF (2003) An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech 36(6):765–776. doi:10.1016/S0021-9290(03)00010-1
Malluche HH, Porter DS, Monier-Faugere M, Mawad H, Pienkowski D (2012) Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol 23(3):525–532. doi:10.1681/ASN.2010121253
Marotti G (2000) The osteocyte as a wiring transmission system. J Musculoskelet Neuronal Interact 1(2):133–136
Marotti G, Zallone AZ (1976) Number, size and arrangement of osteoblasts in osteons at different stages of formation. Calcif Tissue Int 21(suppl):96
Martelli S, Pivonka P, Ebeling PR (2014) Femoral shaft strains during daily activities: implications for atypical femoral fractures. Clin Biomech 29(8):869–876. doi:10.1016/j.clinbiomech.2014.08.001
Martin RB (1972) Effects of geometric feedback in osteoporosis. J Biomech 5:447–455
Martin RB (1984) Porosity and specific surface of bone. Crit Rev Biomed Eng 10(3):179–222
Martin TJ (2004) Paracrine regulation of osteoclast formation and activity: milestones in discovery. J Musculoskelet Neuronal Interact 4(3):243–253
McNamara LM, Prendergast PJ (2007) Bone remodelling algorithms incorporating both strain and microdamage stimuli. J Biomech 40(6):1381–1391
Mittlmeier T, Mattheck C, Dietrich F (1994) Effects of mechanical loading on the profile of human femoral diaphyseal geometry. Med Eng Phys 16:75–81. doi:10.1016/1350-4533(94)90014-0
Morin C, Hellmich C (2014) A multiscale poromicromechanical approach to wave propagation and attenuation in bone. Ultrasonics 54(5):1251–1269. doi:10.1016/j.ultras.2013.12.005
Mosekilde L (1990) Consequences of the remodeling process for vertebral trabecular bone-structure: a scanning electron-microscopy study (uncoupling of unloaded structures). Bone Miner 10:13–35
Mullender MG, Huiskes R, Weinans H (1994) A physiological approach to the simulation of bone remodeling as a self-organizational control process. J Biomech 27(11):1389–1394
Murdock J (1996) Mechanics of Fluids. In: McGraw-Hill (ed) Mark’s standard handbook for mechanical engineers, 10th edn, McGraw-Hill, pp 3.29–3.61
Nordin BEC, Need AG, Chatterton BE, Horowitz M, Morris HA (1988) The relative contributions of age and years since menopause to postmenopausal bone loss. J Clin Endocrinol Metab 70(1):83–88
Parfitt A (1983) The physiologic and clinical significance of bone histomorphometric data. In: Recker R (ed) Bone histomorphometry: techniques and interpretation, chap 9. CRC Press, Boca Raton, pp 143–224
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2(6):595–610. doi:10.1002/jbmr.5650020617
Parfitt A (1998) A structural approach to renal bone disease. J Bone Miner Res 13(8):1213–1220
Parfitt A, Chir B (1987) Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obstet Gynecol 30(4):789–811
Pettermann H, Reiter T, Rammerstorfer F (1997) Computational simulation of internal bone remodeling. Archiv Comput Methods Eng 4(4):295–323
Pivonka P, Zimak J, Smith DW, Gardiner BS, Dunstan CR, Sims NA, Martin TJ, Mundy GR (2008) Model structure and control of bone remodeling: a theoretical study. Bone 43(2):249–263. doi:10.1016/j.bone.2008.03.025
Pivonka P, Zimak J, Smith DW, Gardiner BS, Dunstan CR, Na Sims, Martin TJ, Mundy GR (2010) Theoretical investigation of the role of the RANK–RANKL–OPG system in bone remodeling. J Theor Biol 262(2):306–316. doi:10.1016/j.jtbi.2009.09.021
Pivonka P, Buenzli PR, Scheiner S, Hellmich C, Dunstan CR (2013) The influence of bone surface availability in bone remodelling: a mathematical model including coupled geometrical and biomechanical regulations of bone cells. Eng Struct 47:134–147
Pivonka P, Buenzli PR, Dunstan CR (2012) A systems approach to understanding bone cell interactions in health and disease. In: Gowder S (ed) Cell interactions, chap. 7
Prendergast PJ, Taylor D (1994) Prediction of bone adaptation using damage accumulation. J Biomech 27(8):1067–1076
Raisz LG, Seeman E (2001) Causes of age-related bone loss and bone fragility: an alternative view. J Bone Miner Res 16(11):1948–1952
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20(2):92–102
Rittweger J, Simunic B, Bilancio G, Gaspare De Santo N, Cirillo M, Biolo G, Pisot R, Eiken O, Mekjavic IB, Narici M (2009) Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone 44(4):612–618. doi:10.1016/j.bone.2009.01.001
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498
Roodman GD (1999) Cell biology of the osteoclast. Exp Hematol 27(8):1229–1241
Ruff CB (2000) Body size, body shape, and long bone strength in modern humans. J Hum Evol 38(2):269–290. doi:10.1006/jhev.1999.0322
Ruimerman R, Van Rietbergen B, Hilbers P, Huiskes R (2005) The effects of trabecular-bone loading variables on the surface signaling potential for bone remodeling and adaptation. Ann Biomed Eng 33(1):71–78
Russo CR, Lauretani F, Seeman E, Bartali B, Bandinelli S, Di Iorio A, Guralnik J, Ferrucci L (2006) Structural adaptations to bone loss in aging men and women. Bone 38:112–118. doi:10.1016/j.bone.2005.07.025
Salencon J (2001) Handbook of continuum mechanics (general concepts, thermoelasticity), 1st edn. Springer, Berlin
Scheiner S, Pivonka P, Hellmich C (2013) Coupling systems biology with multiscale mechanics, for computer simulations of bone remodeling. Comput Methods Appl Mech Eng 254:181–196. doi:10.1016/j.cma.2012.10.015
Skerry TM, Bitensky L, Chayen J, Lanyon LE (1988) Loading-related reorientation of bone proteoglycan in vivo. Strain memory in bone tissue? J Orthop Res 6(4):547–551
Squire M, Brazin A, Keng Y, Judex S (2008) Baseline bone morphometry and cellular activity modulate the degree of bone loss in the appendicular skeleton during disuse. Bone 42(2):341–349. doi:10.1016/j.bone.2007.09.052
Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD (2006) Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21(12):1856–1863. doi:10.1359/jbmr.060904
Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J (2007) Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone 41:745–751. doi:10.1016/j.bone.2007.07.019
Thomas CDL, Feik SA, Clement JG (2005) Regional variation of intracortical porosity in the midshaft of the human femur: age and sex differences. J Anat 206(2):115–125. doi:10.1111/j.1469-7580.2005.00384.x
Timoshenko S, Goodier JN (1951) Theory of elasticity. McGraw-Hill, New York
Turner CH, Robling AG, Duncan RL, Burr DB (2002) Do bone cells behave like a neuronal network? Calcif Tissue Int 70(6):435–442
Van der Meulen MCH, Beaupre GS, Carter DR (1993) Mechanobiologic influences in long bone cross-sectional growth. Bone 14(4):635–642
Van Oers RFM, Ruimerman R, Tanck E, Hilbers PAJ, Huiskes R (2008) A unified theory for osteonal and hemi-osteonal remodeling. Bone 42(2):9–250. doi:10.1016/j.bone.2007.10.009
Van Rietbergen B, Müller R, Ulrich D, Rüegsegger P, Huiskes R (1999) Tissue stresses and strain in trabeculae of a canine proximal femur can be quantified from computer reconstructions. J Biomech 32(4):51–443
Vaughan CL, Davis BL, O’Connor JC (1992) Dynamics of human gait. Kiboho Publishers, Cape Town
Viceconti M, Testi D, Taddei F, Martelli S, Clapworthy GJ, Jan S (2006) Biomechanics modeling of the musculoskeletal apparatus : status and key issues. Proc IEEE 94(4):725–739
Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehailia M, Alexandre C (2000) Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355(9215):1607–1611. doi:10.1016/S0140-6736(00)02217-0
Wang FS, Wang CJ, Chen YJ, Huang YT, Huang HC, Chang PR, Sun YC, Yang KD (2004) Nitric oxide donor increases osteoprotegerin production and osteoclastogenesis inhibitory activity in bone marrow stromal cells from ovariectomized rats. Endocrinology 145:2148–2156
Warner SE, Sanford DA, Becker BA, Bain SD, Srinivasan S, Gross TS (2006) Botox induced muscle paralysis rapidly degrades bone. Bone 38:257–264
Weinans H, Huiskes R, Grootenboer HJ (1992) The behavior of adaptive bone-remodeling simulation models. J Biomech 25(12):1425–1441
Weinbaum S, Cowin S, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27(3):339–360. doi:10.1016/0021-9290(94)90010-8
Weiner S, Wagner HD (1998) The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28(1):271–298
Weyts F, Bosmans B, Niesing R, Van Leeuwen J, Weinans H (2003) Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcif Tissue Int 72(4):505–512. doi:10.1007/s00223-002-2027-0
Zaoui A (1997) Structural morphology and constitutive behavior of microheterogeneous materials. In: Suquet P (ed) Continuum Micromech. Springer, Wien, pp 291–347
Zaoui A (2002) Continuum micromechanics: survey. J Eng Mech 128(August):808–816
Zebaze RMD, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375(9727):1729–1736. doi:10.1016/S0140-6736(10)60320-0
Acknowledgments
We thank C. David L. Thomas and Prof. John G. Clement for providing the microradiographs of the femur cross sections. PRB is the recipient of an Australian Research Council Discovery Early Career Research Award (DE130101191).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Appendices
Appendix 1: Complements on the model description
1.1 Differentiation rates and signalling molecules in the cell populations model
In Sect. 2.3, we presented the simplified equations of the bone cells population model. Here are the developments of these equations.
In those equations, several signalling molecules play a role: \(\text {TGF} \upbeta \), RANK, RANKL, OPG, MCSF and PTH. The concentrations of these molecules follow the principles of mass action kinetics of receptor–ligand reactions. Due to the separation of scale between the cells differentiation and apoptosis rates and the receptor–ligand binding reactions, we solve them in a quasi-steady-state hypothesis:

1.2 Model parameter values
Table 1 lists all the parameters of the model.
Most model parameters were calibrated and validated in previous studies. The stiffness tensors \(\mathbbm {c}^{\text {micro}}_\text {bm}\) and \(\mathbbm {c}^{\text {micro}}_\text {vas}\) arising in the micromechanical theory were measured by mechanical experiments (Ashman et al. 1984; Murdock 1996); see also (Fritsch and Hellmich 2007). The secretory rate \(\text {k}_{\mathrm{form}}\)(volume of bone matrix formed per osteoblast per day) was measured in animal models (Marotti and Zallone 1976; Jones 1974). The value used here is based on osteonal data from dogs, rescaled to match human osteon dimensions (Buenzli et al. 2014). The resorption rate \(k_\text {res}\) (volume of bone matrix resorbed per osteoclast per day) was estimated based on the progression speed of a cortical BMU in bone and the average number of \(\text {OC}_{\mathrm{a}}\) per BMU (Buenzli et al. 2014).
The \(\text {OB}_{\mathrm{a}}\) and \(\text {OC}_{\mathrm{a}}\) cell densities are calibrated in our model to give rise to site-specific turnover rates in steady state in agreement with experimental measurements (see Sect. 2.3). The parameters \(\lambda \) and \(\kappa \) related to the strength of mechanical adaptation and the parameter \(\text {P}^{\text {OP}}_{\text {PTH}}\) related to the rate of bone loss during osteoporosis were calibrated in Sect. 2.6 based on experimental data.
The densities and dynamical behaviours of the precursor cell types (\(\text {OB}_{\mathrm{p}}\), \(\text {OB}_{\mathrm{u}}\), \(\text {OC}_{\mathrm{p}}\) and \(\text {OC}_{\mathrm{u}}\)) and of the biochemical signalling molecules (\(\text {TGF} \upbeta \), PTH, RANK, RANKL, OPG and MCSF) depend on all the remaining parameters, which represent binding reaction rates, differentiation rates, proliferation rates and apoptosis rates (see Table 1). These parameters were validated qualitatively in previous studies (Pivonka et al. 2008; Buenzli et al. 2012; Pivonka et al. 2013), i.e. they give rise to physiologically expected qualitative behaviours of the model. A quantitative calibration of these parameters to physiological values of precursor cell densities and molecule concentrations was not done due to the lack of experimental data. However, such a quantitative calibration is not an issue since it can be done without altering the time evolution of the model so long as the densities of the active cells (\(\text {OC}_{\mathrm{a}}\), \(\text {OB}_{\mathrm{a}}\)) remain properly calibrated in Sect. 2.3. Indeed, in our simulations, both the signalling molecules and precursor cell densities (\(\text {OC}_{\mathrm{u}}\), \(\text {OC}_{\mathrm{p}}\), \(\text {OB}_{\mathrm{u}}\), \(\text {OB}_{\mathrm{p}}\)) are effectively in a quasi-steady at all times, the former by model assumption (see Sect. 6.1) and the latter due to the slow evolution of bone adaptation (years) compared to the faster bone cell dynamics (days, weeks). To increase the value of density or concentration of a component by a factor \(\alpha \), one may increase its production rate by the factor \(\alpha \) or reduce its elimination rate by the factor \(1/\alpha \). To maintain the system’s behaviour with this new value of density or concentration, it suffices to adjust the sensitivity of interactions with this component with the relevant parameters (such as binding reaction rate parameters). For example, to double the concentration of \(\text {TGF} \upbeta \) without affecting how it signals \(\text {OC}_{\mathrm{a}}\), \(\text {OB}_{\mathrm{u}}\) and \(\text {OB}_{\mathrm{p}}\), one may double \(n^\text {bone}_{\text {TGF} \upbeta }\) or halve \(D_{\text {TGF} \upbeta }\) (see Eq. (25)) and then double \(k^{\text {TGF} \upbeta }_{\text {OC}_{\mathrm{a}}}\), \(k^{\text {TGF} \upbeta }_{\text {OB}_{\mathrm{u}}}\) and \(k^{\text {TGF} \upbeta }_{\text {OB}_{\mathrm{p}}}\) so that the ratios \(\text {TGF} \upbeta /k^{\text {TGF} \upbeta }_{\text {OC}_{\mathrm{a}}}\), \(\text {TGF} \upbeta /k^{\text {TGF} \upbeta }_{\text {OB}_{\mathrm{u}}}\) and \(\text {TGF} \upbeta /k^{\text {TGF} \upbeta }_{\text {OB}_{\mathrm{p}}}\) in Eq. (23) have the same values. A similar approach was used in Buenzli et al. (2014) to calibrate the cell populations within a single BMU. This approach is also used in Sect. 6.3 below.
1.3 Recalibration of the model
Since \(\text {OC}_{\mathrm{u}}\) and \(\text {OB}_{\mathrm{u}}\) vary with \({f_\text {bm}}\) so as to retrieve experimentally valid turnover rates, some other parameters required modification compared with previous versions of the cell population model in which \(\text {OC}_{\mathrm{u}}\) and \(\text {OB}_{\mathrm{u}}\) were constant and uncalibrated (Buenzli et al. 2012; Pivonka et al. 2013).
By comparing the cell densities between this model and the previously published one (Buenzli et al. 2012), we can determine scaling coefficients which allows a systematic calibration of \(\pi ^\text {act}\big (\tfrac{\text {TGF} \upbeta }{k^{\text {TGF} \upbeta }_{\text {OC}_{\mathrm{a}}}}\big )\) and \(\pi ^\text {act}\big (\tfrac{\text {RANKL}}{k^{\text {RANKL}}_{\text {OC}}}\big )\). Indeed, these functions depend on the active and precursor cell densities. In the original models, the constants in these functions were calibrated such as to obtain a strong biochemical feedback response. Maintaining this strong biochemical response is the aim of this re-calibration.
The calibration is realised at \({f_\text {bm}}\) = 0.90. Both the turnover rate value and the values of \(k_\text {res}\) and \(\text {k}_{\mathrm{form}}\) have been changed according to the literature. Hence, by isolating \(\text {OB}_{\mathrm{a}}\) and \(\text {OC}_{\mathrm{a}}\) in the two new constraints of the steady state, the active osteoblast and active osteoclast read:
if \(\delta \) is the coefficient of proportionality between the new bone turnover rate and the previous one, \(\beta = \delta \cdot k_\text {res}/ k_{\text {res}}^{\text {new}}\) and \(\gamma = \delta \cdot \text {k}_{\mathrm{form}}/ k_{\text {form}}^{\text {new}}\). These coefficients are introduced in the determination of \(\text {TGF} \upbeta \) and \(\text {OPG}\). Previously, \(\text {TGF} \upbeta \) was (Buenzli et al. 2012):
The new one becomes:
The same manipulation is realised on the determination of OPG. The factor \(\beta ^{\text {OPG}}_{\text {OB}_{\text {a}}}\, \text {OB}_{\text {a}}\) in Eq. (27) becomes \(\beta ^{\text {OPG}}_{\text {OB}_{\text {a}}}\, \text {OB}_{\text {a}}^{\text {new}} \gamma ^{-1}\).
Appendix 2: Update frequency of mechanical state in the numerical algorithm
In our model, to simulate osteoporosis and the change of porosity with time, we need to solve the temporal equations of the bone cell populations model, Eqs. (14)–(17) and Eq. (12). Those equations via the mechanical feedback are correlated with the spatial Eqs. (2) and (42). Knowing the porosity distribution is required to determine the stress and strain distributions. Hence, we have a semi-coupled algorithm (Fig. 2).
However, due to the separation of time scale, we can decompose the problem into two parts. Indeed, it takes more time for the microstructure to change significantly enough to influence the bone cell populations model. Therefore, we solve the bone cell populations model for a duration \(\varDelta \)t, assuming the mechanical feedback to be constant in this time interval. Then, we recalculate the stress and strain distributions based on the new porosity distribution, and this becomes the new mechanical feedback.
A sensitivity analysis of the solution in the time step \(\varDelta \)t of evolution of cell densities and bone matrix volume fraction is required. For very small time steps (\(\varDelta \)t \(\le \) 1 day), one would expect the algorithm to converge to the exact solution. On the other hand for very large time steps (\(\varDelta \)t \(\ge \) 5 years), a large deviation from the exact solution is expected. Figure 11 shows the evolution of the bone matrix volume fraction for one selected RVE (y = 0, z = \(-10\) mm) in the cross section. These simulations show that time steps of \(\varDelta \)t = 250 days, 1 and 2 years lead to very similar evolution of the bone matrix volume fraction. On the other hand, \(\varDelta \)t = 5 years and 10 years lead to strong deviations from the smaller time increments. For all the simulations of 40 years of osteoporosis, we used a time step of 2 years.
Appendix 3: Generalised Beam theory for inhomogeneous materials
In the following, we represent the governing equations using a Cartesian (x, y, z) coordinate system. The x-axis represents the beam axis and coincides with the direction of the vascular pores (i.e. Haversian systems). The y and z coordinates describe a material point in the cross section (Fig. 1c). The origin of the system is known as the normal force centre: NC. Since our cross sections are inhomogeneous all the quantities, including the stiffness, are functions of y and z.
First, based on the constitutive relation: Hooke’s law, we determine the strain and stress relation:
where \({\varvec{\sigma }}^\text {tissue}(y, z, t)\) and \({\varvec{\varepsilon }}^\text {tissue}(y, z, t)\) are the “tissue” stress and strain and \(\mathbb {C}^\text {tissue}(y, z, t)\) the tissue stiffness matrix. The stiffness matrix is determined at the tissue scale, and the explanation is presented in Sect. 2.1.
Based on the Bernoulli hypothesis, the strain distribution appears to be a plane and remains plane even after deformation. This is why we can decompose the strain by introducing three constants: \(\varepsilon _1\), \(\kappa _3\) and \(\kappa _2\).
By introducing this relation into Hooke’s law, we obtain:
Because we assume the shear force to be null, the stress tensor is reduced to one component: \(\sigma ^\text {tissue}_{xx}(y, z, t)\). And with Bernoulli hypothesis, the strain tensor contains only one component. Hence, the stiffness matrix can be replaced by the component \(\mathbb {C}^\text {tissue}_{xx}(y, z, t)\).
Here we can see that if we determine the strain constants, we would know the stress distribution. The mechanical loadings, the inputs of this model, allow us to determine the strain. Indeed, the cross section is supposed to be under a normal force: \({\varvec{N}}\) and a bending moment \({\varvec{M}}\) here divided in two bending moments: \(M_y\) and \(M_z\), such as \(M ~\hat{{\varvec{m}}} = M_y ~\hat{{\varvec{y}}} + M_z ~\hat{{\varvec{z}}}\). By definition of the stress, we have the relations:
By introducing the static moments of first and second order: EA, \(ES_{y}\), \(ES_{z}\), \(EI_{yy}\), \(EI_{zz}\), \(EI_{yz}\), the equations become the following constitutive relation:
where:
If we chose the origin of the coordinate system at the normal centre (NC) of the cross section, the coupling terms between extension and bending vanish since they become null by definition of the NC:
The constitutive relation can be simplified as:
1.1 Determination of the normal force centre NC
The special location of the origin of the coordinate system for which the coupling terms (\(ES_{y}\) and \(ES_{z}\)) between extension and bending become zero is by definition called the normal force centre NC. The result of this definition is that an axial force \({\varvec{N}}\) which acts at the NC only causes straining and no bending. The coupling terms are also referred to as weighted static moments or weighted first order moments. To find the position of the coordinate system for which the coupling terms become zero requires a tool.
Assume a temporary coordinate system: \(\overline{y} - \overline{z}\) from which the porosity distribution is known. The shift in origin of this coordinate system with respect to the y–z coordinate system through the unknown NC is denoted with \(\overline{y}_{NC}\) and \(\overline{z}_{NC}\). The temporary coordinate system can be expressed in terms of the y–z coordinate system as:
Hence:
By definition, \(ES_{y}\) and \(ES_{z}\) with respect to the y–z coordinate system are zero from which the unknown position of the NC with respect to the known position of the \(\overline{y}\)– \(\overline{z}\) coordinate system can be found:
To conclude, here is the step-by-step methodology we are using to find the stress and strain distribution in the cross section:
-
1.
Localise the normal centre (NC) by computing the integrations: EA, \(ES_{y}\), \(ES_{z}\).
-
2.
Compute the integrations: \(EI_{yy}\), \(EI_{zz}\) and \(EI_{yz}\).
-
3.
Determine the cross-sectional forces: \({\varvec{N}}\), \(M_{y}\) and \(M_{z}\).
-
4.
Calculate the cross-sectional deformations: \(\varepsilon _1\), \(\kappa _2\) and \(\kappa _3\) based on Eq. (42).
-
5.
Find the strain distribution based on the kinematic relation, Eq. (34). Here it is important to remember to use the coordinate centred in NC.
-
6.
Find the stress distribution based on Hooke’s law, Eq. (33).
The initial cross section is extracted from a microradiograph, as it is explained in Sect. 2.4; the mechanical loading is not symmetrical. Hence, the position of the NC is changing. This is why we need to localise it after each step.
Rights and permissions
About this article
Cite this article
Lerebours, C., Buenzli, P.R., Scheiner, S. et al. A multiscale mechanobiological model of bone remodelling predicts site-specific bone loss in the femur during osteoporosis and mechanical disuse. Biomech Model Mechanobiol 15, 43–67 (2016). https://doi.org/10.1007/s10237-015-0705-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0705-x