Abstract
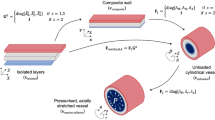
Despite increasing experimental and analytical efforts to investigate the irreversible effects of arterial tissue failure, the underlying mechanisms are still poorly understood. The goal of this study was to characterize the failure properties of the intact wall and each separated layer (intima, media, and adventitia) of the descending thoracic and infrarenal abdominal aorta and to test the hypothesis that the failure properties of layer-separated tissue depend on the location of the aorta. To test this hypothesis, we performed uniaxial tests to study the mechanical behavior of both intact and layer-separated porcine aortic tissue samples taken from descending thoracic and infrarenal abdominal aorta until complete failure. The fracture stress is higher in the infrarenal abdominal aorta than in the equivalent descending thoracic aorta. It was also found that the extrapolation of the elastic mechanical properties from the physiological to the supra-physiological regime for characterizing the mechanical response of the aorta would be inappropriate. Finally, we report values of constitutive parameters using phenomenological and microstructural damage models based on continuum damage mechanics theory. The phenomenological damage model gives an excellent fit to the experimental data compared to the microstructural damage model. Although the fitting results of the phenomenological model are better, the microstructural models can include physically motivated aspects obtained from experiments.










Similar content being viewed by others
References
Alastrué V et al (2009) Anisotropic micro-sphere-based finite elasticity applied to blood vessel modelling. J Mech Phys Solids 57:178–203
Alastrué V et al (2008) Experimental study and constitutive modelling of the passive mechanical properties of the ovine infrarenal vena cava tissue. J Biomech 41:3038–3045
Alastrué V et al (2010) On the use of bingham statistical distribution in microsphere-based constitutive models for arterial tissue. Mech Res Commun 37:700–706
Balzani D et al (2006) Simulation of discontinuous damage incorporating residual stress in circumferentially overstretched atherosclerotic arteries. Acta Biomater 2:609–618
Barra JG et al (1993) Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res 73:1040–1050
Bažant P, Oh BH (1986) Efficient numerical integration on the surface of a sphere. ZAMM-Z Angew Math Mech 66:37–49
Calvo B et al (2007) An uncoupled directional damage model for fibered biological soft tissues: formulation and computational aspects. Int J Numer Meth Eng 69:2036–2057
Carew TE et al (1968) Compressibility of the arterial wall. Circ Res 23:61–86
Demiray H (1972) A note on the elasticity of soft biological tissues. J Biomech 5:309–311
Diani J et al (2009) A review on the Mullins effect. Eur Polym J 45:601–612
Duprey A et al (2016) Biaxial rupture properties of ascending thoracic aortic aneurysms. Acta Biomat 42:273–285
Ehret AE, Itskov M (2009) Modeling of anisotropic softening phenomena: application to soft biological tissues. Int J Plasticity 25:901–919
Eppell SJ et al (2006) Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. J R Soc Interface 3:117–121
Ferrara A, Pandolfi A (2010) A numerical study of arterial media dissection processes. Int J Fract 166:21–33
Flory PJ (1961) Thermodynamic relations for high elastic materials. Trans Faraday Soc 57:829–838
Forsell C, Gasser TC (2011) Numerical simulation of the failure of ventricular tissue due to deeper penetration, the impact of constitutive properties. J Biomech 44:45–51
Fung YC (1993) Biomechanics. Mechanical properties of living tissues, 2nd edn. Springer, New York
García A et al (2013) Determination and modeling of the inelasticity over the length of the porcine carotid artery. ASME J Biomech Eng 135:031004-1
Gasser TC (2011) An irreversible constitutive model for fibrous soft biological tissue: a 3-D microfiber approach with demonstrative application to abdominal aortic aneurysms. Acta Biom 7:2457–2466
Gasser TC et al (2012) Spatial orientation of collagen fibers in the abdominal aortic aneurysm’s wall and its relation to wall mechanics. Acta Biomat 8:3091–3103
Gasser TC, Holzapfel GA (2006) Modeling the propagation of arterial dissection. Eur J Mech A/Solids 25:617–633
Gasser TC et al (2006) Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface 3:15–35
Gültekin O et al (2016) A phase-field approach to model fracture of arterial walls: theory and finite element analysis. Comput Meth Appl Mech Eng 312:542–566
Guo X, Kassab GS (2003) Variation of mechanical properties along the length of the aorta in C57bl/6 mice. Am J Physiol Heart Circ Physiol 285:H2614–H2622
Hamedzadeh A et al (2018) On the constitutive modelling of recruitment and damage of collagen fibres in soft biological tissues. Eur J Mech A/Solids 72:483–496
Hang HC, Fung YC (1995) Longitudinal strain of canine and porcine aortas. J Biomech 28:637–641
Hernández Q, Peña E (2016) Failure properties of vena cava tissue due to deep penetration during filter insertion. Biomech Model Mechanobiol 15:845–856
Hokanson J, Yazdami S (1997) A constitutive model of the artery with damage. Mech Res Commun 24:151–159
Holzapfel GA et al (2005) Determination of the layer-specific mechanical properties of human coronary arteries with non-atherosclerotic intimal thickening, and related constitutive modelling. Am J Physiol Heart Circ Physiol 289:H2048–H2058
Holzapfel GA et al (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elasticity 61:1–48
Humphrey JD (2002) Cardiovascular solid mechanics: cells, tissues, and organs. Springer, New York
Hurschler C et al (1997) A structurally based stress-stretch relationship for tendon and ligament. ASME J Biomech Eng 119:392–399
Kamenskiy AV et al (1998) Biaxial mechanical properties of the human thoracic and abdominal aorta, common carotid, subclavian, renal and common iliac arteries. Biomech Model Mechanobiol 13:1341–1359
Kim J, Baek S (2011) Circumferential variations of mechanical behavior of the porcine thoracic aorta during inflation test. J Biomech 44:1941–1947
Kim J et al (2013) Longitudinal differences in the mechanical properties of the thoracic aorta depend on circumferential regions. J Biomed Mater Res Part A 101:1525–1529
Kim J-H et al (2012) Experimental characterization of rupture in human aortic aneurysms using a full-field measurement technique. Biomechan Model Mechanobiol 11:841–853
Lally C et al (2004) Elastic Behavior of Porcine Coronary Artery Tissue under Uniaxial and Equibiaxial Tension. Ann Biomed Eng 32:1355–1364
Lebedev VI, Laikov DN (1999) A quadrature formula for the sphere of the 131st algebraic order of accuracy. Doklady Math 59:477–481
Li D, Robertson AM (2009) A structural multi-mechanism damage model for cerebral arterial tissue. ASME J Biomech Eng 131(101013):1–8
Lillie MA et al (2012) Contribution of elastin and collagen to the inflation response of the pig thoracic aorta: assessing elastin’s role in mechanical homeostasis. J Biomech 45:2133–2141
Lu X et al (2003) Shear modulus of porcine coronary artery: contributions of media and adventitia. Am J Physiol Heart Circ Physiol 285:H1966–H1975
Maher E et al (2012) Site specific inelasticity of arterial tissue. J Biomech 45:1393–1399
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. Siam J Appl Math 11:431–441
Mullins L (1947) Effect of stretching on the properties of rubber. Rubber Res 16:275–289
Noble C et al (2016) Creating a model of diseased artery damage and failure from healthy porcine aorta. J Mech Behav Biomed 60:378–393
Nolan DR, McGarry JP (2016) On the compressibility of arterial tissue. Ann Biomed Eng 44:993–1007
Peña E (2011a) A rate dependent directional damage model for fibred materials: application to soft biological tissues. Comp Mech 48:407–420
Peña E (2011b) Damage functions of the internal variables for soft biological fibred tissues. Mech Res Commun 38:610–615
Peña E (2011c) Prediction of the softening and damage effects with permanent set in fibrous biological materials. J Mech Phys Solids 59:1808–1822
Peña E (2014) Computational aspects of the numerical modelling of softening, damage and permanent set in soft biological tissues. Comput Struct 130:57–72
Peña E et al (2010) A constitutive formulation of vascular tissue mechanics including viscoelasticity and softening behaviour. J Biomech 43:984–989
Peña E, Doblare M (2009) An anisotropic pseudo-elastic approach for modelling Mullins effect in fibrous biological materials. Mech Res Commun 36:784–790
Peña E et al (2009) On the Mullins effect and hysteresis of fibered biological materials: a comparison between continuous and discontinuous damage models. Int J Solids Struct 46:1727–1735
Peña JA et al (2018) Over length quantification of the multiaxial mechanical properties of the ascending, descending and abdominal aorta using Digital Image Correlation. J Mech Behav Biomed 77:434–445
Peña JA et al (2015) Layer-specific residual deformations and uniaxial and biaxial mechanical properties of thoracic porcine aorta. J Mech Behav Biomed 50:55–69
Pierce DM et al (2015) Human thoracic and abdominal aortic aneurysmal tissues: Damage experiments, statistical analysis and constitutive modeling. J Mech Behav Biomed 41:92–107
Polzer S et al (2015) Structure-based constitutive model can accurately predict planar biaxial properties of aortic wall tissue. Acta Biomater 14:133–145
Raina A, Miehe C (2016) A phase-field model for fracture in biological tissues. Biomech Model Mechanobiol 15:479–496
Rajagopal K, et al (2019) The mechanics of acute aortic dissection: measured calculations and calculated measures. J Thorac Cardiovasc Surg
Rezakhaniha R et al (2012) Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol 11:461–473
Rodríguez JF et al (2006) A stochastic-structurally based three dimensional finite-strain damage model for fibrous soft tissue. J Mech Phys Solids 54:564–886
Schriefl A et al (2012) Determination of the layer-specific distributed collagen fiber orientations in human thoracic and abdominal aortas and common iliac arteries. J R Soc Interface 9:1275–1286
Sáez P et al (2012) Anisotropic microsphere-based approach to damage in soft fibered tissue. Biomechan Model Mechanobiol 11:595–608
Sáez P et al (2016) Microstructural quantification of collagen fiber orientations and its integration in constitutive modeling of the porcine carotid artery. Acta Biomat 33:183–193
Silver FH et al (2003) Mechanical behavior of vessel wall: a comparative study of aorta, vena cava, and carotid artery. Ann Biomed Eng 31:793–803
Simo JC (1987) On a fully three-dimensional finite-strain viscoelastic damage model: formulation and computational aspects. Comput Methods Appl Mech Eng 60:153–173
Sokolis DP (2010) A passive strain-energy function for elastic and muscular arteries: correlation of material parameters with histological data. Med Biol Eng Comput 48:507–518
Spencer AJM (1971) Theory of invariants continuum physics. Academic Press, New York, pp 239–253
Volokh KY (2007a) Hyperelasticity with softening for modeling materials failure. J Mech Phys Solids 55:2237–2264
Volokh KY (2007b) Prediction of arterial failure based on a microstructural bi-layer fiber-matrix model with softening. J Biomech 41:447–453
Weisbecker H et al (2012) Layer-specific damage experiments and modeling of human thoracic and abdominal aortas with non-atherosclerotic intimal thickening. J Mech Behav Biomed 12:93–106
Weisbecker H et al (2015) Constitutive modelling of arteries considering fibre recruitment and three-dimensional fibre distribution. J R Soc Interface 12:20150111
Wulandana R, Robertson AM (2005) An inelastic multi-mechanism constitutive equation for cerebral arterial tissue. Biomech Model Mechanbiol 4:235–248
Zeinali-Davarani S et al (2013) Characterization of biaxial mechanical behavior of porcine aorta under gradual elastin degradation. Ann Biomed Eng 41:1528–1538
Acknowledgements
Financial support for this research was provided by the Spanish Ministry of Economy and Competitiveness through Research Project DPI2016-76630-C2-1-R; the Department of Industry and Innovation (Government of Aragon) through the research group Grant T24-17R (Fondo Social Europeo); and the Instituto de Salud Carlos III (ISCIII) through the CIBER initiative. The work was performed by the ICTS “NANBIOSIS” specifically by the Tissue and Scaffold Characterization Unit (U13), of the CIBER in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN at the University of Zaragoza).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peña, J.A., Martínez, M.A. & Peña, E. Failure damage mechanical properties of thoracic and abdominal porcine aorta layers and related constitutive modeling: phenomenological and microstructural approach. Biomech Model Mechanobiol 18, 1709–1730 (2019). https://doi.org/10.1007/s10237-019-01170-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-019-01170-0