Abstract
Patients with chronic obstructive pulmonary disease (COPD) often suffer from other conditions, such as cardiovascular disease, that further increase the risk of adverse outcomes in this group. Serum homocysteine concentrations are positively associated with cardiovascular risk and have also been reported to be increased in COPD. This meta-analysis investigated the association between homocysteine concentrations and COPD. A systematic search of publications in the electronic databases PubMed, Web of Science, Scopus, and Google Scholar, from inception to September 2021, was conducted using the following terms: “Homocysteine” or “Hcy” and “Chronic Obstructive Pulmonary Disease” or “COPD”. Weighted mean differences (WMDs) were calculated to evaluate differences in homocysteine concentrations between COPD patients and non-COPD subjects. Risk of bias and certainty of evidence were assessed using the Joanna Briggs Institute Critical Appraisal Checklist and GRADE, respectively. Nine studies in 432 COPD patients (mean age 65 years, 65% males) and 311 controls (mean age 65 years, 56% males) were identified. Pooled results showed that serum homocysteine concentrations were significantly higher in patients with COPD (WMD = 2.91 µmol/L, 95% CI 2.00–3.82 µmol/L; p < 0.001; high certainty of evidence). No publication bias was observed. Our results support the hypothesis that increased homocysteine concentrations are significantly associated with COPD and may account, at least in part, for the increased cardiovascular risk in these patients.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide [1]. In 2017, there were ~ 270 million cases of COPD and ~ 18 million were newly reported globally [2]. COPD is an inflammatory condition characterized by persistent respiratory symptoms and airflow obstruction due to both small airways disease and parenchymal destruction [3]. The airflow limitation is progressive and not completely reversible and appears to be the result of a chronic inflammatory state that worsens with the progression of the disease [4, 5]. Cigarette smoking and other irritants stimulate macrophages and airway epithelial cells to release mediators of inflammation that are responsible for the recruitment of inflammatory cells. In this context, an important role is also played by oxidative stress, a condition where reactive oxygen species (ROS) overcome the antioxidant defence systems [6, 7]. Inflammatory cells release ROS that activate proinflammatory molecules, leading to the recruitment of further inflammatory cells. Moreover, ROS activate NF-κB which, in turn, activates several inflammatory genes and impairs the function of antiproteases, such as α1-antitrypsin, accelerating the breakdown of elastin in the lung parenchyma [4, 6]. The prognosis of patients with COPD is often complicated by the presence of comorbidities [8, 9]. For example, co-existing cardiovascular disease leads to high rates of morbidity and risk of hospitalisation and mortality in patients with COPD [10]. It is widely established that elevated serum or plasma concentrations of the sulfur-containing amino acid homocysteine, an intermediate in the metabolism of the essential amino acid methionine, increase cardiovascular risk [11,12,13]. Homocysteine causes endothelial dysfunction through the induction of inflammation and oxidative stress, both involved in the pathogenesis of COPD, and the inhibition of nitric oxide synthesis [14,15,16]. Homocysteine concentrations can increase mainly as the result of specific genetic defects of the enzymes responsible for homocysteine metabolism as well as deficiencies of cofactors involved in this pathway such as vitamins B6, B12 and folic acid. Such deficiencies are secondary to reduced intake or absorption and use of specific medications [17, 18]. Recent studies have shown that homocysteine concentrations are also increased in COPD patients, particularly in those with severe disease and rapid progression [19, 20]. Therefore, to critically appraise the available evidence on the link between homocysteine and COPD, we conducted a systematic review and meta-analysis of homocysteine concentrations in serum or plasma of COPD patients and in subjects without COPD.
Materials and methods
Search strategy, eligibility criteria, and study selection
A systematic search of publications in the electronic databases PubMed, Web of Science, Scopus and Google Scholar, from inception to September 2021, was conducted using the combination of the following terms: “Homocysteine” or “Hcy” and “Chronic Obstructive Pulmonary Disease” or “COPD”. Two investigators screened the abstracts to establish relevance and, if so, the full text was reviewed according to the following inclusion criteria: (i) assessment of serum homocysteine concentrations; (ii) comparison of subjects with and without COPD (case–control design); (iii) adult participants; (iv) English language; and (v) full text available. The references of the retrieved articles were also searched for additional studies. Any discordance between the two investigators was resolved by a third investigator. The risk of bias was evaluated using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for analytical cross-sectional studies, with scores ≥ 5, 4 and < 4 indicating low, moderate and high risk, respectively [21]. The certainty of evidence was assessed using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group system. GRADE considers the study design (randomized vs. observational), the risk of bias (JBI checklist), the presence of unexplained heterogeneity, the indirectness of the evidence, the imprecision of the results (sample size, 95% confidence interval width and threshold crossing), the effect size (WMD) and a high probability of publication bias [22,23,24]. The assessment of the potential biological and clinical significance of the WMD was based on prospective studies reporting that a 10% increase in homocysteine concentrations is associated with an adjusted hazard ratio for cardiovascular events between 1.87 and 1.97 [25]. For a baseline homocysteine concentration of 15 µmol/L, the WMD was therefore considered high (> − 1.5 µmol/L, 10% reduction), moderate (between − 0.75 and − 1.5 µmol/L, 5–10% reduction) and low (< − 0.75 µmol/L, < 5% reduction), respectively. We fully complied with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statement on the reporting of systematic reviews and meta-analyses [26].
Statistical analysis
Weighted mean differences (WMDs) and 95% confidence intervals (95% CIs) were calculated to generate forest plots of continuous data and to evaluate differences in homocysteine concentrations between COPD patients vs. non-COPD subjects (a p-value of less than 0.05 was considered statistically significant). When necessary, the mean and standard deviation values were extrapolated from median and interquartile range values, as previously reported by Wan et al. [27] or from graphs using the Graph Data Extractor software. Heterogeneity of WMD values across studies was assessed using the Q-statistic (significance level set at a p-value of less than 0.10). The I2 statistic, a quantitative measure of inconsistency across studies, was also calculated with values < 25% indicating no heterogeneity, between 25 and 50% moderate heterogeneity, between 50 and 75% large heterogeneity and > 75% extreme heterogeneity) [28, 29]. Statistical heterogeneity was defined as an I2 statistic value ≥ 50% [29]. In analyses in which heterogeneity was high, a random-effects model was used. Sensitivity analysis was conducted to investigate the influence of individual studies on the overall risk estimate by excluding them sequentially [30]. To evaluate the presence of publication bias, the associations between study size and magnitude of effect were analyzed using the Begg’s adjusted rank correlation test and the Egger’s regression asymmetry test, with a significance level set at a p-value of less than 0.05 [31, 32]. Statistical analyses were performed using Stata 14 (STATA Corp., College Station, TX, USA). The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42021282004).
Results
Systematic research and study characteristics
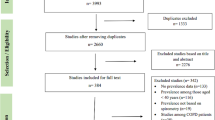
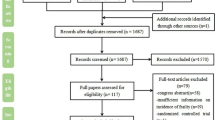
A flow chart describing the screening process is presented in Fig. 1. We initially identified 910 studies. A total of 898 were excluded after the first screening because they were either duplicates or irrelevant. After a full text review of the remaining 12 articles, three were further excluded because they did not meet the inclusion criteria. Therefore, nine studies in 432 COPD patients (mean age 65 years, 65% males) and 311 controls (mean age 65 years, 56% males), published between 2001 and 2020, were included in the final analysis (Table 1) [19, 20, 33,34,35,36,37,38,39]. In eight studies, the diagnosis of COPD was made according to the Global Obstructive Lung Disease (GOLD) guidelines [19, 20, 34,35,36,37,38,39]. No information regarding the tool used for COPD diagnosis was provided in the remaining study [33].
Risk of bias
The risk of bias was considered low in all studies (Table 2).
Results of individual studies and syntheses
The forest plot for serum homocysteine concentrations in COPD patients and non-COPD subjects is shown in Fig. 2. In all studies, COPD patients had higher homocysteine concentrations compared to non-COPD subjects with a significant difference reported in all but one [38]. A large heterogeneity between studies was observed; thus, random-effects models were used. Overall, pooled results showed that homocysteine concentrations were significantly higher in patients with COPD (WMD = 2.91 µmol/L, 95% CI 2.00–3.82 µmol/L, p < 0.001). Sensitivity analysis showed that the corresponding pooled WMD values were not substantially altered when each study was sequentially removed (Fig. 3). Funnel plot analysis showed three outliers (Fig. 4). After removing these studies [34, 36, 38], the WMD remained significant (WMD = 2.68 µmol/L, 95% CI 1.89–3.87 µmol/L, p < 0.001) but with a virtually absent heterogeneity (Fig. 5).
Sensitivity analysis of the association between serum homocysteine and COPD. The influence of individual studies on the overall weighted mean difference (WMD) is shown. The middle vertical axis indicates the overall WMD, and the two vertical axes indicate the 95% confidence intervals (CIs). The hollow circles represent the pooled WMD when the remaining study is omitted from the meta-analysis. The two ends of each broken line represent the 95% CIs
Publication bias
No publication bias was observed either with the Begg’s (p = 0.47) or the Egger’s (p = 0.55) test.
Certainty of evidence
The initial level of certainty for homocysteine WMD values was considered low because of the cross-sectional nature of the studies (rating 2, ⊕ ⊕ ⊝ ⊝). After considering the low risk of bias in all studies (no rating change required), a generally extreme heterogeneity that was virtually eliminated after removing a subgroup of three studies (no rating change required), the lack of indirectness (no rating change required), the relatively low imprecision (relatively narrow confidence intervals without threshold crossing, upgrade one level), the relatively large effect size (upgrade one level) and the absence of publication bias (no rating change required), the overall level of certainty was considered high (rating 4, ⊕ ⊕ ⊕ ⊕).
Discussion
This is an updated systematic review and meta-analysis that reports for the first time a significant association between homocysteine concentrations and the presence of COPD. Mild-to-moderate hyperhomocysteinemia is relatively common in the general population, especially in older adults, and is mainly associated with deficiencies in group B vitamins, such as vitamin B12, vitamin B6 and folic acid which serve as cofactors in homocysteine metabolism [17, 18]. As COPD is also characterized by complex nutritional deficiencies [40], it is plausible that the presence of a poor folic acid status may lead to elevated homocysteine concentrations in these patients. This hypothesis, however, requires further research as a study failed to provide evidence that hyperhomocysteinemia in COPD patients represented a reliable marker of folate and vitamin B12 deficiencies [41]. Nevertheless, another study showed a reduction of homocysteine concentrations in COPD patients after folate supplementation [38]. Moreover, several epidemiological studies have reported significant and positive associations between homocysteine concentrations and cardiovascular disease, an important comorbidity that also shares some risk factors with COPD, such as cigarette smoking and increasing age which are also associated with high homocysteine concentrations [17]. The results of our study further support the presence of an association between homocysteine and COPD. A similar meta-analysis, published in 2019 by Chaudhary et al. [42], included only four studies on 145 COPD patients and 107 non-COPD subjects and failed to report significant between-group differences in homocysteine concentrations. Our study included a greater number of articles, nine, and subjects, 432 COPD patients and 311 non-COPD subjects. The overall result showed the presence of significantly higher concentrations of homocysteine in COPD patients compared to non-COPD subjects. The high heterogeneity observed may be explained by several unreported factors, such as differences in sample handling, storage conditions and analytical procedures as well as geographical location, ethnicity, smoking status and presence of comorbidities. Specifically, three studies contributed to the reported heterogeneity. Their removal virtually eliminated the heterogeneity while maintaining significant between-group differences in homocysteine. Additional limitations include the lack of subgroup analysis to identify specific associations between effect size and other clinical or analytical characteristics, as this information was not provided. Despite these limitations, this meta-analysis reports an association between homocysteine and COPD presence with a high level of certainty and without publication bias. Moreover, in sensitivity analysis, the pooled WMD values were not substantially altered when individual studies were sequentially removed. Some of the studies also reported that homocysteine concentration was positively related to COPD severity [19, 20]. However, their limited number prevented the conduct of additional pooled analyses. Our findings support the hypothesis that increased homocysteine concentrations are associated with COPD and that such association may at least partially account for the increased cardiovascular risk in these patients. In recent studies, plasma concentrations of homocysteine > 10 µmol/L have been shown to be associated with the development of cardiovascular disease [43]. Shiao et al. reported that for each 5 μmol/L increase in homocysteine, the risk of mortality increased by 32%, and the risk of heart disease increased by 52% [44]. Another meta‐analysis showed that a 3 µmol/L decrease in serum homocysteine concentrations was associated with a 16% reduction in coronary heart disease and that a 5 µmol/L increase was associated with a 1.6–1.8 fold risk of coronary heart disease [45]. Several experimental and human studies have shown that homocysteine may disrupt endothelial and vascular homeostasis by causing increased vascular smooth muscle cell proliferation, oxidative damage, and alterations of coagulation [46].
In conclusion, our updated systematic review and meta-analysis has shown that COPD patients have significantly higher homocysteine concentrations when compared to non-COPD controls. This might at least partially explain the common occurrence of atherosclerotic cardiovascular disease in COPD patients given the well-known deleterious effects of homocysteine on endothelial function and vascular homeostasis. However, further research is warranted to investigate the presence of additional factors mediating the association between homocysteine and COPD and whether interventions targeting this highly reactive amino acid improve respiratory and cardiovascular outcomes in this group.
Change history
28 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–128.
Viegi G, Maio S, Fasola S, Baldacci S. Global burden of chronic respiratory diseases. J Aerosol Med Pulm Drug Deliv. 2020;33:171–7.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–82.
Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27.
Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, Wouters EF. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076.
McGuinness AJ, Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms. J Clin Med. 2017;6:21.
Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144:266–73.
Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–12.
Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology. 2015;20:1160–71.
Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: Dangerous liaisons? Eur Respir Rev. 2018;27:180057.
Selhub J, Troen AM. Sulfur amino acids and atherosclerosis: a role for excess dietary methionine. Ann N Y Acad Sci. 2016;1363:18–25.
Givvimani S, Qipshidze N, Tyagi N, Mishra PK, Sen U, Tyagi SC. Synergism between arrhythmia and hyperhomo-cysteinemia in structural heart disease. Int J Physiol Pathophysiol Pharmacol. 2011;3:107–19.
Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–22.
Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6.
Djuric D, Jakovljevic V, Zivkovic V, Srejovic I. Homocysteine and homocysteine-related compounds: an overview of the roles in the pathology of the cardiovascular and nervous systems. Can J Physiol Pharmacol. 2018;96:991–1003.
Jiang Q, Wang L, Si X, Tian JL, Zhang Y, Gui HL, Li B. Tan DH current progress on the mechanisms of hyperhomocysteinemia-induced vascular injury and use of natural polyphenol compounds. Eur J Pharmacol. 2021;905:174168.
Al MF. Hyperhomocysteinemia: clinical insights. J Cent Nerv Syst Dis. 2020;12:1179573520962230.
Kluijtmans LA, Young IS, Boreham CA, Murray L, McMaster D, McNulty H, Strain JJ, McPartlin J, Scott JM, Whitehead AS. Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood. 2003;101:2483–8.
Zinellu A, Zinellu E, Sotgiu E, Fois AG, Paliogiannis P, Scano V, Piras B, Sotgia S, Mangoni AA, Carru C, Pirina P. Systemic transsulfuration pathway thiol concentrations in chronic obstructive pulmonary disease patients. Eur J Clin Invest. 2020;7:e13267.
Wei B, Tian T, Liu Y, Li C. The diagnostic value of homocysteine for the occurrence and acute progression of chronic obstructive pulmonary disease. BMC Pulm Med. 2020;20:237.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute reviewer’s manual. Adelaide: Johanna Briggs Institute; 2017.
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, et al. The GRADE working group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13.
Zhang Y, Coello PA, Guyatt GH, Yepes-Nunez JJ, Akl EA, Hazlewood G, Pardo-Hernandez H, Etxeandia-Ikobaltzeta I, Qaseem A, Williams JW, et al. GRADE guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences-inconsistency, imprecision, and other domains. J Clin Epidemiol. 2019;111:83–93.
Veeranna V, Zalawadiya SK, Niraj A, Pradhan J, Ference B, Burack RC, Jacob S, Afonso L. Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol. 2011;58:1025–33.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–7.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55.
Andersson A, Ankerst J, Lindgren A, Larsson K, Hultberg B. Hyperhomocysteinemia and changed plasma thiol redox status in chronic obstructive pulmonary disease. Clin Chem Lab Med. 2001;39:229–33.
Kai S, Nomura A, Morishima Y, Ishii Y, Sakamoto T, Hegab AE, Sekizawa K. The effect of smoking-related hyperhomocysteinemia on spirometric declines in chronic obstructive pulmonary disease in elderly Japanese. Arch Gerontol Geriatr. 2006;42:117–24.
Seemungal TA, Lun JC, Davis G, Neblett C, Chinyepi N, Dookhan C, Drakes S, Mandeville E, Nana F, Setlhake S, et al. 2011 Plasma homocysteine is elevated in COPD patients and is related to COPD severity. Int J Chron Obstr Pulmon Dis. 2007;2(3):313–21.
Abdallah GM, Abdullah A, Omran GA, Mariee AD. Serum homocystein level in COPD patients: possible beneficial effect of antioxidants. Res J Med Med Sci. 2009;4:306–10.
Fimognari FL, Loffredo L, Di Simone S, Sampietro F, Pastorelli R, Monaldo M, Violi F, D’Angelo A. Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr Metab Cardiovasc Dis. 2009;19:654–9.
Khan NA, Saini H, Mawari G, Kumar S, Hira HS, Daga MK. The effect of folic acid supplementation on hyperhomocysteinemia and pulmonary function parameters in chronic obstructive pulmonary disease. A pilot study. J Clin Diagn Res. 2016;10:oc17–21.
Moayyedkazemi A, Rahimirad MH. Evaluating the serum homocysteine level in the patients with chronic obstructive pulmonary disease and its correlation with severity of the disease. J Res Med Dent Sci. 2018;6:123–6.
Schols AM. Nutritional and metabolic modulation in chronic obstructive pulmonary disease management. Eur Respir J. 2003;22:81Se6.
Beletić A, Mirković D, Dudvarski-Ilić A, Milenković B, Nagorni-Obradović L, Đorđević V, Ignjatović S, Majkić-Singh N. Questionable reliability of homocysteine as the metabolic marker for folate and vitamin B12 deficiency in patients with chronic obstructive pulmonary disease. J Med Biochem. 2015;34:467–72.
Chaudhary D, Sharma N, Senapati S. Serum homocysteine could be used as a predictive marker for chronic obstructive pulmonary disease: a meta-analysis. Front Public Health. 2019;4(7):69.
Ostrakhovitch E, Tabibzadeh S. Homocysteine and age-associated disorders. Ageing Res Rev. 2019;49:144–64.
Shiao SPK, Lie A, Yu CH. Meta-analysis of homocysteine-related factors on the risk of colorectal cancer. Oncotarget. 2018;9:25681.
Ntaios George. Homocysteine, B vitamins, and cardiovascular risk. In: Foods and dietary supplements in the prevention and treatment of disease in older adults. Amsterdam: Elsevier; 2015.
Mishra N. Hyperhomocysteinemia: a risk of CVD. Int J Res Biol Sci. 2016;6:13–9.
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement. This research was supported by grants from the Sardinian Fondo di Sviluppo e Coesione (FSC) 2014—2020, Patto per lo Sviluppo della Regione Sardegna, LR7-2017 (RASSR82005) and Fondo di Ateneo per la Ricerca- annualità 2020.
Author information
Authors and Affiliations
Contributions
Conceptualization, AZ. and PP.; methodology, AZ., EZ. and AAM.; Data curation and investigation: EZ., MCP., SM., BP., VS., and SSF; formal analysis: AZ., EZ., AGF. and CC.; original draft preparation, AZ., EZ. and PP.; review and editing, AZ., EZ., AGF., AAM., CC. and PP.; funding acquisition, AZ., and C.C.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zinellu, A., Zinellu, E., Pau, M.C. et al. A systematic review and meta-analysis of homocysteine concentrations in chronic obstructive pulmonary disease. Clin Exp Med 23, 751–758 (2023). https://doi.org/10.1007/s10238-022-00833-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00833-0