Abstract
Purpose
Patients suffering from cardiovascular autonomic failure often develop neurogenic supine hypertension (nSH), i.e., high blood pressure (BP) in the supine position, which falls in the upright position owing to impaired autonomic regulation. A committee was formed to reach consensus among experts on the definition and diagnosis of nSH in the context of cardiovascular autonomic failure.
Methods
As a first and preparatory step, a systematic search of PubMed-indexed literature on nSH up to January 2017 was performed. Available evidence derived from this search was discussed in a consensus expert round table meeting in Innsbruck on February 16, 2017. Statements originating from this meeting were further discussed by representatives of the American Autonomic Society and the European Federation of Autonomic Societies and are summarized in the document presented here. The final version received the endorsement of the European Academy of Neurology and the European Society of Hypertension.
Results
In patients with neurogenic orthostatic hypotension, nSH is defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, measured after at least 5 min of rest in the supine position. Three severity degrees are recommended: mild, moderate and severe. nSH may also be present during nocturnal sleep, with reduced-dipping, non-dipping or rising nocturnal BP profiles with respect to mean daytime BP values. Home BP monitoring and 24-h-ambulatory BP monitoring provide relevant information for a customized clinical management.
Conclusions
The establishment of expert-based criteria to define nSH should standardize diagnosis and allow a better understanding of its epidemiology, prognosis and, ultimately, treatment.
Similar content being viewed by others
Introduction
Primary cardiovascular autonomic failure develops in the context of inherited and sporadic neurodegenerative diseases affecting the autonomic nervous system. Secondary causes of cardiovascular autonomic failure include amyloidosis and metabolic or immune-mediated diseases inducing autonomic neuropathy [1].
The main feature of cardiovascular autonomic failure is neurogenic orthostatic hypotension (nOH), defined in 2011 as a sustained reduction of systolic blood pressure (BP) of ≥ 20 mmHg (≥ 30 mmHg in patients with supine hypertension) or diastolic BP of ≥ 10 mmHg within 3 min of standing or head-up tilt of at least 60° [2]. About one-half of the patients with nOH develop neurogenic supine hypertension (nSH), which can be severe and last several hours during sleep [3]. nSH is distinct from essential hypertension, since most patients with nSH are normotensive while seated and may be severely hypotensive while standing [4,5,6].
As yet, no consensus criteria have been established for the diagnosis of nSH, limiting current understanding of its epidemiology, prognostic significance and the development of appropriate management strategies.
To address this shortcoming, a round table discussion with participants from the European Federation of Autonomic Societies (EFAS) and from the American Autonomic Society (AAS) was convened in Innsbruck on February 16, 2017 to establish clinical criteria for the diagnosis of nSH in the context of cardiovascular autonomic failure.
Discussion points and statements originating from this meeting were subsequently examined and reviewed by representatives of both societies and are summarized in the document presented here. The final version was endorsed by the European Academy of Neurology (EAN) and by the European Society of Hypertension (ESH).
Definitions
Supine hypertension
In patients with proven nOH, nSH is defined as systolic BP of ≥ 140 mmHg and/or diastolic BP of ≥ 90 mmHg, measured after at least 5 min of rest in the supine position.
We propose the following ranges to define the severity of nSH in autonomic failure:
-
Mild nSH: systolic BP values of 140–159 mmHg or diastolic BP values of 90–99 mmHg.
-
Moderate nSH: systolic BP values of 160–179 mmHg or diastolic BP values of 100–109 mmHg.
-
Severe nSH: systolic BP values of ≥ 180 mmHg or diastolic BP values of ≥ 110 mmHg.
Nocturnal hypertension
Patients with cardiovascular autonomic failure frequently show nSH also during sleep, i.e. nocturnal hypertension, with loss of the physiological nocturnal BP fall at night of ≥ 10% while supine and asleep (dipping). Two main pathological nocturnal BP profiles are distinguished:
-
Reduced-dipping: characterized by a mean nocturnal BP reduction of < 10% with respect to mean daytime BP values.
-
Non-dipping or rising: when the mean BP does not decrease or even increases during the night with respect to daytime [7, 8].
Additional aspects are important for an appropriate interpretation of 24 h-ambulatory BP monitoring (24 h-ABPM) in patients with cardiovascular autonomic failure and are discussed in the section Diagnostic work-up.
Clinical features
Neurogenic supine hypertension is mostly asymptomatic or induces only non-specific complaints, such as headache. The main, most clinically relevant and immediate consequence of nSH is an exacerbation of pressure natriuresis during sleep, causing nocturia, sleep disturbances, volume depletion overnight and worsening of nOH in the morning [9, 10].
nSH could potentially result in hypertensive emergencies, with similar complications as in the general population, i.e. cerebral hemorrhage, ischemic stroke, acute pulmonary edema and myocardial infarction [11], although the overall occurrence of these clinical events has not been systematically investigated in cardiovascular autonomic failure. Acute cardiovascular complications associated with nSH have been reported, but mainly in patients also receiving anti-hypotensive drugs [12, 13], which are known to induce or exacerbate nSH (Fig. 1).

Adapted from Fanciulli et al. [44], with permission of Springer SBM. This image is excluded from the creative commons license
Nocturnal blood pressure (BP) profiles at 24 h-ambulatory BP monitoring a Physiological nocturnal dipping profile (mean nocturnal BP falls by ≥ 10% with respect to daytime BP), b reduced-dipping profile (mean nocturnal BP falls by < 10% with respect to daytime BP), c rising profile (mean nocturnal BP increases with respect to daytime BP), in a patient with cardiovascular autonomic failure. Note severe hypotension occurring in the early morning. HR Heart rate. The blue area indicates the pulse pressure, the green line indicates the mean BP.
The long-term effects of cardiovascular autonomic failure have been studied in patients with Parkinson’s disease (PD) and multiple system atrophy (MSA). Current evidence suggests that early-onset cardiovascular autonomic failure is associated with a poor prognosis [14,15,16,17,18,19,20], development of cardiovascular [21,22,23], kidney [24] and cerebrovascular [25,26,27,28,29,30] disease, as well as cognitive impairment [26, 27, 31,32,33,34,35,36]. Similarly, a higher prevalence of left ventricular hypertrophy, nephropathy and vascular and cerebrovascular disease has been reported in diabetic patients with secondary cardiovascular autonomic failure compared to those without [37].
At present, it is uncertain how much of the increase in risk is related to the underlying disease causing autonomic failure and how much is directly related to the abnormality in BP regulation. It is also unclear whether nOH or nSH represents the actual negative prognostic factor in patients with cardiovascular autonomic failure—or whether it is the BP volatility induced by the combination of both [38].
Epidemiology
Keeping in mind the limitations due to inconsistences in the diagnostic criteria, nSH has been reported in 34–46% of patients with PD and 37% of patients with MSA [3, 39]. The frequency rates of nSH increase to 50% in parkinsonian patients with nOH, supporting the assumption of an etiological association between nOH and nSH [3, 39,40,41,42]. Loss of nocturnal BP dipping has been frequently reported in PD (48%) and MSA patients (up to 75%) [43,44,45,46].
The prevalence of nSH in pure autonomic failure (PAF) ranges from 48 to 70% [24, 47]. An abnormal, non-dipping, nocturnal BP profile has been described in up to 86% of patients with PAF [48].
To date, no studies have addressed the epidemiology of nSH in diabetes, but a significant association has been reported between the presence of nOH and rising nocturnal BP profiles both in patients with type 1 and type 2 diabetes mellitus [49, 50].
The prevalence of nSH in genetic and acquired amyloidosis, as well as in other kinds of autonomic neuropathies is unknown. Clinically, patients with acquired amyloidosis (AL) and nOH often present with relatively low supine BP measurements which may be related to cardiovascular amyloid deposition.
Pathophysiology
Anti-hypotensive drugs used for the treatment of nOH may unmask or exacerbate nSH, thereby posing a management challenge. However, nSH can also develop in the absence of anti-hypotensive treatment, which reflects the likelihood that multiple factors contribute to nSH, including impairment of the afferent, central and efferent pathways of the arterial baroreflex arch, disruption of the renin–angiotensin–aldosterone axis and denervation supersensitivity at the level of the vascular adrenoceptors due to impaired sympathetic transmission [6, 41, 51,52,53].
Noradrenaline release from intact post-ganglionic sympathetic fibers in the setting of severe baroreflex impairment drives nSH in MSA, which is characterized in most cases by severe preganglionic sympathetic denervation, with sparing of the post-ganglionic sympathetic fibers to the heart and blood vessels [53].
In contrast, mechanisms independent of the sympathetic autonomic nervous system are considered to contribute to the development of nSH in autonomic disorders with post-ganglionic sympathetic denervation and very low circulating noradrenaline levels, such as in PAF [53]. Paradoxically increased angiotensin II and aldosterone receptor signaling in the setting of decreased activity of the systemic renin–angiotensin–aldosterone system has been implicated [51, 54]. In contrast, an impairment in nitric oxide-mediated vasodilation has not been observed in patients with nSH due to primary cardiovascular autonomic failure [55].
Diagnostic work-up
All patients newly diagnosed with nOH should be screened for nSH at the time of diagnosis and at regular intervals afterwards, especially if they begin treatment with anti-hypotensive agents, increase their dosage, report multiple episodes of nocturia per night or develop ankle edema.
Office screening should be performed by measuring supine BP as soon as the patient assumes the supine position and then again after the patient has been resting supine for at least 5 min. This can be combined with a standing test, by having the patient stand immobile to avoid engaging the muscle pumps in the lower limbs, or with a tilt table examination. A tilt table examination may be preferred in patients unable to stand for several minutes.
It should be noted that the criteria outlined here to define nSH only apply for patients with proven nOH: if present, both conditions are usually verified during the same test. Exceptions to this rule may be due to the fact that nOH and nSH could manifest with different degrees of severity according to the time of the day, intravascular volume status and timing of the administration of anti-hypotensive drugs. Anti-hypotensive drugs may succeed in raising the standing BP and improving symptoms, but exacerbate nSH.
A diagnosis of nSH should be also made independently from seated BP values, which may vary from hypertensive values to normo- or hypotensive values in patients with cardiovascular autonomic failure.
In addition to office BP screening, home BP recordings performed by patients themselves are recommended to gather further insights into circadian BP control. Although no protocol for home BP self-monitoring has been validated in patients with cardiovascular autonomic failure, we recommend that the BP be recorded three times per day (early morning, after lunch, at bedtime) in the supine, seated and standing position for 1 week at first diagnostic work-up. The same home BP protocol should be repeated after the initiation or adaptation of any treatment for nOH or nSH in order to monitor therapeutic effects and to detect potential side effects. Comparison of supine and seated BP values during the daytime is particularly valuable for choosing therapeutic strategies: patients with nSH may show normal BP values while seated, hence benefitting from a simple non-pharmacological approach such as avoiding the supine position during daytime.
If office BP screening or home BP recordings suggest nSH, a 24 h-ABPM is recommended to ascertain the presence of nocturnal hypertension and document absolute BP levels reached overnight. 24 h-ABPM may also provide information on the severity of nOH during daily activity or after meals (“post-prandial hypotension” [56]), thus supporting customization of the therapeutic management [57].
Additional considerations are required when interpreting 24 h-ABPM data of patients with cardiovascular autonomic failure:
-
Several activities of daily life may cause severe drops in BP, which may be captured in the 24-h recordings. For an accurate interpretation of the 24 h-ABPM data, patients should be instructed to compile an activity diary on the examination day in which they report the times of medication intake (especially anti-hypotensive and anti-hypertensive agents), meals, physical activities and times of getting out of bed at night (for example, to use the bathroom).
-
Extreme drops in BP during daytime will reduce mean BP during the daytime, thereby resulting in an overestimation of the percentage of nocturnal BP rise. Conversely, nocturnal standing or bathroom visits may induce nocturnal BP falls, which may result in an underestimation of the BP rise overnight. For these reasons, both the percentage of nocturnal BP rise, as well as the absolute overnight BP values should be considered when diagnosing and tailoring treatment strategies for nSH. Whereas non-dipping and rising nocturnal patterns have been associated with long-term end-organ damage, absolute BP values reached while supine may be more relevant for the development of hypertensive emergencies.
-
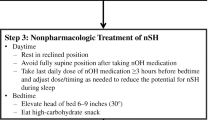
Sleeping with the bed 12° head-up tilted (or higher, if tolerated) creates an overnight gravitational stress and is an effective non-pharmacological strategy to manage nOH [58]. Sleeping with the whole bed tilted can mitigate the severity of nocturnal nSH by inducing venous pooling below the level of the heart throughout the night. Sleeping with only the head of the bed raised appears to be less effective in lowering night-time BP. Close attention should be therefore paid to the angle of the bed when interpreting the 24 h-ABPM data.
-
Patients with cardiovascular autonomic failure, especially in the setting of PD and MSA, may suffer from sleep fragmentation or sleep disordered breathing, both of which have been associated with development of hypertension [59,60,61]. If sleep disordered breathing is suspected at clinical history taking or based on 24 h-ABPM data (for example, by documenting multiple nocturnal hypertensive peaks), a targeted diagnostic work-up is indicated.
-
Nocturnal BP profiles may change over the short term with repeated 24 h-ABPM in 10–35% patients with essential hypertension [62, 63]. Reproducibility of the 24 h-ABPM readings in patients with cardiovascular autonomic failure remains to be investigated. This may be important as BP can fluctuate on a day-to-day basis due to multiple factors.
Finally, if a nocturnal BP rise is documented in a patient not known to suffer from any autonomic disorder, screening for nOH may be indicated. Similarly, if a patient is found to have elevated BP while supine—for example, in a hospital or acute care setting—they should also have the BP measured seated and, if the systolic difference exceeds 10 mmHg, standing as well. Otherwise, nSH might be mistaken for essential hypertension, and if treatment decisions are based on supine BP values alone, medications could potentially worsen unrecognized nOH.
Perspectives
Current epidemiological data are not sufficient to define cut-offs for nSH based on its impact on end-organ damage or mortality. For this reason, the criteria proposed here for the diagnosis of nSH in the context of cardiovascular autonomic failure are based on the criteria for the diagnosis of essential hypertension and interpretation of 24 h-ABPM recordings in the general population [7, 8]. There are several reasons supporting this strategy: first, to facilitate the use of these cut-off values in clinical practice; second, to apply severity degrees of nSH as a tool for customizing treatment and grade adverse events in future interventional clinical trials for nOH; third, to generate data which can be compared across other epidemiological cohorts, such as essential hypertension. These definitions of nSH should ultimately lead to a better understanding of the prognostic outcome and stratification of the risk of adverse events.
References
Benarroch EE (2014) The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol 10(7):396–407
Freeman R, Wieling W, Axelrod FB et al (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21(2):69–72
Fanciulli A, Gobel G, Ndayisaba JP et al (2016) Supine hypertension in Parkinson’s disease and multiple system atrophy. Clin Auton Res 26(2):97–105
Cicolini G, Pizzi C, Palma E et al (2011) Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens 24(10):1073–1079
Krzesinski P, Stanczyk A, Gielerak G, Piotrowicz K, Banak M, Wojcik A (2016) The diagnostic value of supine blood pressure in hypertension. Arch Med Sci 12(2):310–318
Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y (2003) Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 42(2):136–142
Parati G, Stergiou G, O’Brien E et al (2014) European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 32(7):1359–1366
Mancia G, Fagard R, Narkiewicz K et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34(28):2159–2219
Wilcox CS, Aminoff MJ, Penn W (1974) Basis of nocturnal polyuria in patients with autonomic failure. J Neurol Neurosurg Psychiatry 37(6):677–684
Jordan J, Shannon JR, Pohar B et al (1999) Contrasting effects of vasodilators on blood pressure and sodium balance in the hypertension of autonomic failure. J Am Soc Nephrol 10(1):35–42
Pinna G, Pascale C, Fornengo P et al (2014) Hospital admissions for hypertensive crisis in the emergency departments: a large multicenter Italian study. PLoS One 9(4):e93542
Sandroni P, Benarroch EE, Wijdicks EF (2001) Caudate hemorrhage as a possible complication of midodrine-induced supine hypertension. Mayo Clin Proc 76(12):1275
Chaimberg KH, Travis KW (2002) Supine hypertension during general anesthesia in a patient taking midodrine. Anesth Analg 95(5):1196–1197 (table of contents)
Cilia R, Cereda E, Klersy C et al (2015) Parkinson’s disease beyond 20 years. J Neurol Neurosurg Psychiatry 86(8):849–855
Stubendorff K, Aarsland D, Minthon L, Londos E (2012) The impact of autonomic dysfunction on survival in patients with dementia with lewy bodies and Parkinson’s disease with dementia. PLoS One 7(10):e45451
CCalandra-Buonaura G, Guaraldi P, Sambati L, et al (2013) Multiple system atrophy with prolonged survival: is late onset of dysautonomia the clue? Neurol Sci 34(10):1875–1878
Petrovic IN, Ling H, Asi Y et al (2012) Multiple system atrophy-parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord 27(9):1186–1190
O’Sullivan SS, Massey LA, Williams DR et al (2008) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131(Pt 5):1362–1372
Tada M, Onodera O, Ozawa T et al (2007) Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol 64(2):256–260
Watanabe H, Saito Y, Terao S et al (2002) Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 125(Pt 5):1070–1083
Maule S, Milan A, Grosso T, Veglio F (2006) Left ventricular hypertrophy in patients with autonomic failure. Am J Hypertens 19(10):1049–1054
Maule S, Milazzo V, Maule MM, Di Stefano C, Milan A, Veglio F (2012) Mortality and prognosis in patients with neurogenic orthostatic hypotension. Funct Neurol 27(2):101–106
Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H (2000) Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet 355(9205):725–726
Garland EM, Gamboa A, Okamoto L et al (2009) Renal impairment of pure autonomic failure. Hypertension 54(5):1057–1061
Oh YS, Kim JS, Lee KS (2013) Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J Mov Disord 6(2):23–27
Pilleri M, Facchini S, Gasparoli E et al (2013) Cognitive and MRI correlates of orthostatic hypotension in Parkinson’s disease. J Neurol 260(1):253–259
Kim JS, Oh YS, Lee KS, Kim YI, Yang DW, Goldstein DS (2012) Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 79(13):1323–1331
Gorell JM, Johnson CC, Rybicki BA (1994) Parkinson’s disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology 44(10):1865–1868
Tha KK, Terae S, Yabe I et al (2010) Microstructural white matter abnormalities of multiple system atrophy: in vivo topographic illustration by using diffusion-tensor MR imaging. Radiology 255(2):563–569
Lim TS, Lee PH, Kim HS, Yong SW (2009) White matter hyperintensities in patients with multiple system atrophy. J Neurol 256(10):1663–1670
Jones JD, Jacobson C, Murphy M, Price C, Okun MS, Bowers D (2014) Influence of hypertension on neurocognitive domains in nondemented Parkinson’s disease patients. Parkinsons Dis 2014:507529
Hohler AD, Zuzuarregui JR, Katz DI et al (2012) Differences in motor and cognitive function in patients with Parkinson’s disease with and without orthostatic hypotension. Int J Neurosci 122(5):233–236
Allcock LM, Kenny RA, Mosimann UP et al (2006) Orthostatic hypotension in Parkinson’s disease: association with cognitive decline? Int J Geriatr Psychiatry 21(8):778–783
Peralta C, Stampfer-Kountchev M, Karner E et al (2007) Orthostatic hypotension and attention in Parkinson’s disease with and without dementia. J Neural Transm 114(5):585–588
Brown RG, Lacomblez L, Landwehrmeyer BG et al (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133(Pt 8):2382–2393
Deguchi K, Takeuchi H, Sasaki I, Tsukaguchi M, Touge T, Nishioka M (2001) Impaired novelty P3 potentials in multiple system atrophy—correlation with orthostatic hypotension. J Neurol Sci 190(1–2):61–67
Milazzo V, Di Stefano C, Milan A et al (2015) Cardiovascular complications in patients with autonomic failure. Clin Auton Res 25(3):133–140
Rothwell PM (2010) Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 375(9718):938–948
Umehara T, Matsuno H, Toyoda C, Oka H (2016) Clinical characteristics of supine hypertension in de novo Parkinson disease. Clin Auton Res 26(1):15–21
Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I (1997) The hypertension of autonomic failure and its treatment. Hypertension 30(5):1062–1067
Biaggioni I, Robertson RM (2002) Hypertension in orthostatic hypotension and autonomic dysfunction. Cardiol Clin 20(2):291–301 (vii)
Pavy-Le Traon A, Piedvache A, Perez-Lloret S et al (2016) New insights into orthostatic hypotension in multiple system atrophy: a European multicentre cohort study. J Neurol Neurosurg Psychiatry 87(5):554–561
Berganzo K, Diez-Arrola B, Tijero B et al (2013) Nocturnal hypertension and dysautonomia in patients with Parkinson’s disease: are they related? J Neurol 260(7):1752–1756
Fanciulli A, Strano S, Ndayisaba JP et al (2014) Detecting nocturnal hypertension in Parkinson’s disease and multiple system atrophy: proposal of a decision-support algorithm. J Neurol 261(7):1291–1299
Plaschke M, Trenkwalder P, Dahlheim H, Lechner C, Trenkwalder C (1998) Twenty-four-hour blood pressure profile and blood pressure responses to head-up tilt tests in Parkinson’s disease and multiple system atrophy. J Hypertens 16(10):1433–1441
Schmidt C, Berg D, Prieur S et al (2009) Loss of nocturnal blood pressure fall in various extrapyramidal syndromes. Mov Disord 24(14):2136–2142
Celedonio JE, Arnold AC, Dupont WD et al (2015) Residual sympathetic tone is associated with reduced insulin sensitivity in patients with autonomic failure. Clin Auton Res 25(5):309–315
Struhal W, Lahrmann H, Mathias CJ (2013) Incidence of cerebrovascular lesions in pure autonomic failure. Auton Neurosci 179(1–2):159–162
Madacsy L, Yasar A, Tulassay T et al (1995) Association of relative nocturnal hypertension and autonomic neuropathy in insulin-dependent diabetic children. Acta Biomed Ateneo Parmense 66(3–4):111–118
Chang J, Hou YP, Wu JL et al (2017) Blood pressure circadian rhythms and adverse outcomes in type 2 diabetics diagnosed with orthostatic hypotension. J Diabetes Investig 9(2):383–388
Arnold AC, Okamoto LE, Gamboa A et al (2016) Mineralocorticoid receptor activation contributes to the supine hypertension of autonomic failure. Hypertension 67(2):424–429
Arnold AC, Okamoto LE, Gamboa A et al (2013) Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension 61(3):701–706
Shannon JR, Jordan J, Diedrich A et al (2000) Sympathetically mediated hypertension in autonomic failure. Circulation 101(23):2710–2715
Arnold AC, Biaggioni I (2012) Management approaches to hypertension in autonomic failure. Curr Opin Nephrol Hypertens 21(5):481–485
Gamboa A, Shibao C, Diedrich A et al (2008) Excessive nitric oxide function and blood pressure regulation in patients with autonomic failure. Hypertension 51(6):1531–1536
Pavelic A, Krbot Skoric M, Crnosija L, Habek M (2017) Postprandial hypotension in neurological disorders: systematic review and meta-analysis. Clin Auton Res 27(4):263–271
Norcliffe–Kaufmann L, Kaufmann H (2014) Is ambulatory blood pressure monitoring useful in patients with chronic autonomic failure? Clin Auton Res 24(4):189–192
van Lieshout JJ, ten Harkel AD, Wieling W (2000) Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res 10(1):35–42
Gama RL, Tavora DG, Bomfim RC, Silva CE, de Bruin VM, de Bruin PF (2010) Sleep disturbances and brain MRI morphometry in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy—a comparative study. Parkinsonism Relat Disord 16(4):275–279
Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F (2002) Sleep disorders and their determinants in multiple system atrophy. J Neurol Neurosurg Psychiatry 72(6):798–800
Calandra-Buonaura G, Provini F, Guaraldi P, Plazzi G, Cortelli P (2016) Cardiovascular autonomic dysfunctions and sleep disorders. Sleep Med Rev 26:43–56
Cuspidi C, Meani S, Valerio C et al (2007) Reproducibility of dipping/nondipping pattern in untreated essential hypertensive patients: impact of sex and age. Blood Press Monit 12(2):101–106
Stenehjem AE, Os I (2004) Reproducibility of blood pressure variability, white-coat effect and dipping pattern in untreated, uncomplicated and newly diagnosed essential hypertension. Blood Press 13(4):214–224
Acknowledgments
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Funding
This was an academic project; no external financial support was allotted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alessandra Fanciulli: nothing to declare. Jens Jordan: served as advisor for Janssen-Cilag, Novartis, Novo-Nordisk and Theravance and received research support from Boston Scientific outside of the submitted work; he is also co-founder of Eternygen GmbH. Italo Biaggioni: receives support from the NIH Rare Disease Clinical Research Network (U54-NS065736), reports consultancies for Lundbeck and Theravance for development of drugs to treat orthostatic hypotension and owns a patent application for an automated inflatable binder for the treatment of orthostatic hypotension. Giovanna Calandra–Buonaura: nothing to declare. William P. Cheshire: nothing to declare. Pietro Cortelli: nothing to declare. Sabine Eschlboeck: nothing to declare. Guido Grassi: nothing to declare. Max J. Hilz: nothing to declare. Horacio Kaufmann: receives support from the NIH Rare Disease Clinical Research Network (U54-NS065736). Heinz Lahrmann: nothing to declare. Giuseppe Mancia: nothing to declare. Gert Mayer: nothing to declare. Lucy Norcliffe–Kaufmann: receives support from the NIH Rare Disease Clinical Research Network (U54-NS065736). Anne Pavy-Le Traon: nothing to declare. Satish R. Raj: receives research support from the Canadian Institutes of Health Research (CIHR; Ottawa, ON, Canada) grant MOP142426 and the Cardiac Arrhythmia Network of Canada (CANet; London, ON, Canada) grants SRG-15-P01-001 and SRG-17-P27-001, and the Vanderbilt Institute for Clinical and Translational Research funded by a Clinical and Translational Science Award from the National Center for Advancing Translational Science from the National Institutes of Health (UL1 TR000445); he also reports consultancies for Lundbeck LLC and GE Healthcare. David Robertson: nothing to declare. Isabel Rocha: nothing to declare. Walter Struhal: nothing to declare. Roland Thijs: receives research support from the Dutch National Epilepsy Fund, ZonMW, NUTS Ohra Fund, Medtronic and AC Thomson Foundation, and has received fees for lectures from Medtronic, UCB and GSK. Konstantinos P. Tsioufis: nothing to declare. J. Gert Van Dijk: nothing to declare. Gregor K. Wenning: nothing to declare.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fanciulli, A., Jordan, J., Biaggioni, I. et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS). Clin Auton Res 28, 355–362 (2018). https://doi.org/10.1007/s10286-018-0529-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-018-0529-8