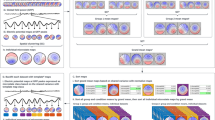
Abstract
Objective
Ultra-high-field functional MRI (UHF-fMRI) allows for higher spatiotemporal resolution imaging. However, higher-resolution imaging entails coverage limitations. Processing partial-coverage images using standard pipelines leads to sub-optimal results. We aimed to develop a simple, semi-automated pipeline for processing partial-coverage UHF-fMRI data using widely used image processing algorithms.
Materials and methods
We developed automated pipelines for optimized skull stripping and co-registration of partial-coverage UHF functional images, using built-in functions of the Centre for Functional Magnetic Resonance Imaging of the Brain's (FMRIB’s) Software library (FSL) and advanced normalization tools. We incorporated the pipelines into the FSL’s functional analysis pipeline and provide a semi-automated optimized partial-coverage functional analysis pipeline (OPFAP).
Results
Compared to the standard pipeline, the OPFAP yielded images with 15 and 30% greater volume of non-zero voxels after skull stripping the functional and anatomical images, respectively (all p = 0.0004), which reflected the conservation of cortical voxels lost when the standard pipeline was used. The OPFAP yielded the greatest Dice and Jaccard coefficients (87 and 80%, respectively; all p < 0.0001) between the co-registered participant gyri maps and the template gyri maps, demonstrating the goodness of the co-registration results. Furthermore, the greatest volume of group-level activation in the most number of functionally relevant regions was observed when the OPFAP was used. Importantly, group-level activations were not observed when using the standard pipeline.
Conclusion
These results suggest that the OPFAP should be used for processing partial-coverage UHF-fMRI data for detecting high-resolution macroscopic blood oxygenation level-dependent activations.






Similar content being viewed by others
References
Yacoub E et al (2001) Imaging brain function in humans at 7 Tesla. Magn Reson Med 45(4):588–594
van der Zwaag W et al (2009) fMRI at 1.5, 3 and 7 T: characterising BOLD signal changes. Neuroimage 47:1425–1434
Duong TQ et al (2003) Microvascular BOLD contribution at 4 and 7 T in the human brain: gradient-echo and spin-echo fMRI with suppression of blood effects. Magn Reson Med 49(6):1019–1027
Gati JS et al (1997) Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med 38(2):296–302
Geissler A et al (2007) Contrast-to-noise ratio (CNR) as a quality parameter in fMRI. J Magn Reson Imaging 25(6):1263–1270
Okada T et al (2005) Magnetic field strength increase yields significantly greater contrast-to-noise ratio increase: measured using BOLD contrast in the primary visual area. Acad Radiol 12(2):142–147
De Martino F et al (2011) Whole brain high-resolution functional imaging at ultra high magnetic fields: an application to the analysis of resting state networks. Neuroimage 57(3):1031–1044
Vu AT et al (2016) Tradeoffs in pushing the spatial resolution of fMRI for the 7 T human connectome project. Neuroimage 154:23–32
Triantafyllou C et al (2005) Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage 26(1):243–250
Yoo PE et al (2017) 7 T-fMRI: Faster temporal resolution yields optimal BOLD sensitivity for functional network imaging specifically at high spatial resolution. Neuroimage 164:214–229
Polimeni JR et al (2010) Laminar analysis of 7 T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 52(4):1334–1346
Huber L et al (2015) Cortical lamina-dependent blood volume changes in human brain at 7 T. Neuroimage 107:23–33
Siero JC et al (2015) Cortical depth dependence of the BOLD initial dip and poststimulus undershoot in human visual cortex at 7 Tesla. Magn Reson Med 73(6):2283–2295
Siero JC et al (2014) BOLD matches neuronal activity at the mm scale: a combined 7 T fMRI and ECoG study in human sensorimotor cortex. Neuroimage 101C:177–184
Yacoub E, Hu X (2001) Detection of the early decrease in fMRI signal in the motor area. Magn Reson Med 45(2):184–190
Yacoub E et al (2001) Investigation of the initial dip in fMRI at 7 Tesla. NMR Biomed 14(7–8):408–412
Yacoub E, Harel N, Ugurbil K (2008) High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci USA 105(30):10607–10612
Yoo PE et al (2018) Spatially dynamic recurrent information flow across long-range dorsal motor network encodes selective motor goals. Hum Brain Mapp 39(6):2635–2650
Polimeni JR et al (2017) Analysis strategies for high-resolution UHF-fMRI data. Neuroimage 168:296–320
Jenkinson M et al (2012) FSL. Neuroimage 62(2):782–790
Avants BB et al (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044
Marques JP et al (2010) MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49(2):1271–1281
Andersson JLR, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20(2):870–888
Smith SM et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143–155
Tustison NJ et al (2014) Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage 99:166–179
Avants BB et al (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54(3):2033–2044
Klein A et al (2010) Evaluation of volume-based and surface-based brain image registration methods. Neuroimage 51(1):214–220
Avants BB et al (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12(1):26–41
Wang XJ (2008) Decision making in recurrent neuronal circuits. Neuron 60(2):215–234
Connolly JD, Andersen RA, Goodale MA (2003) FMRI evidence for a ‘parietal reach region’ in the human brain. Exp Brain Res 153(2):140–145
Medendorp WP et al (2005) Integration of target and effector information in human posterior parietal cortex for the planning of action. J Neurophysiol 93(2):954–962
Bremmer F et al (2001) Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29(1):287–296
Cunnington R et al (2006) The selection of intended actions and the observation of others’ actions: a time-resolved fMRI study. Neuroimage 29(4):1294–1302
Nachev P et al (2005) Volition and conflict in human medial frontal cortex. Curr Biol 15(2):122–128
Sumner P et al (2007) Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54(5):697–711
Binkofski F et al (1999) A parieto-premotor network for object manipulation: evidence from neuroimaging. Exp Brain Res 128(1–2):210–213
Deecke L (1987) Bereitschaftspotential as an indicator of movement preparation in supplementary motor area and motor cortex. Ciba Found Symp 132:231–250
Cunnington R, Bradshaw JL, Iansek R (1996) The role of the supplementary motor area in the control of voluntary movement. Hum Mov Sci 15:627–647
Cunnington R et al (2002) The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage 15(2):373–385
Picard N, Strick PL (2001) Imaging the premotor areas. Curr Opin Neurobiol 11(6):663–672
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443
Wright GA, Hu BS, Macovski A (1991) 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. J Magn Reson Imaging 1(3):275–283
Triantafyllou C et al (2016) Coil-to-coil physiological noise correlations and their impact on functional MRI time-series signal-to-noise ratio. Magn Reson Med 76(6):1708–1719
Felician O et al (2004) The role of human left superior parietal lobule in body part localization. Ann Neurol 55(5):749–751
Gerstmann J (1942) Problem of imperception of disease and of impaired body territories with organic lesions. Arch Neurol Psychiatr 48:890–913
Guariglia C et al (2002) Is autotopoagnosia real? EC says yes. Neuropsychologia 40(10):1744–1749
Felician O et al (2003) Pointing to body parts: a double dissociation study. Neuropsychologia 41(10):1307–1316
Acknowledgements
This work was supported by US Defense Advanced Research Projects Agency (DARPA) Microsystems Technology Office contract N66001-12-1-4045; Office of Naval Research (ONR) Global N62909-14-1-N020; National Health and Medical Research Council of Australia (NHMRC) project grant APP1062532 and development grant APP1075117; Defence Health Foundation, Australia (booster grant); and Defence Science Institute, Australia, grant. Author P.E.Y. acknowledges the Faculty of Medicine, University of Melbourne for the Leslie Eric Paddle Scholarship in Neurology and the Melbourne Neuroscience Institute for the Strategic Australian Postgraduate Award. Author J.O.C was funded by the University of Melbourne McKenzie Fellowship. Author B.A.M acknowledges the Australian National Imaging Facility (NIF) fellowship. We acknowledge the facilities and the scientific and technical assistance of the NIF at the Melbourne Brain Centre Imaging Unit.
Author information
Authors and Affiliations
Contributions
P.E.Y conceived and designed the study, acquired the data, and performed the analyses. P.E.Y, B.A.M, J.O.C, and S.C.K interpreted the data. P.E.Y drafted the manuscript. J.O.C, S.C.K, N.L.O, R.J.O, T.J.O, S.E.J, T.J.O, and B.A.M provided critical revision of the manuscript. T.J.O and B.A.M are joint last authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Thomas J. Oxley and Bradford A. Moffat are joint last authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoo, P.E., Cleary, J.O., Kolbe, S.C. et al. Optimized partial-coverage functional analysis pipeline (OPFAP): a semi-automated pipeline for skull stripping and co-registration of partial-coverage, ultra-high-field functional images. Magn Reson Mater Phy 31, 621–632 (2018). https://doi.org/10.1007/s10334-018-0690-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-018-0690-z