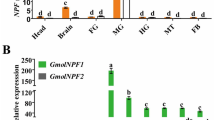
Abstract
Henosepilachna vigintioctopunctata is a highly polyphagous pest of solanaceous plants in Asia and has been predominantly controlled using synthetic insecticides. The intensive use of insecticides has led to the development of resistance, creating an urgent need for new control strategies, for this insect pest. RNA interference (RNAi) has been explored as a pest management strategy in multiple insect pests. In this study, we explored RNAi as a potential alternative for controlling H. vigintioctopunctata. Based on preliminary research, we hypothesized that HvCOPI, a coat protein complex that facilitates retrograde transport, is a promising novel molecular target for H. vigintioctopunctata control. To test this overarching hypothesis, we examined the toxicity of double-stranded RNAs (dsRNAs) towards H. vigintioctopunctata using dietary RNAi toxicity assays targeting eight HvCOPI subunits, including α-, β-, β′-, γ-, δ-, ε-, ζ-, and Arf1-COPI using in vitro synthesized and bacterially expressed dsRNAs. The results demonstrated that ingestion of dsHvCOPIs induced acute feeding cessation in both larvae and adults, which led to significant mortality. In this study, H. vigintioctopunctata showed similar sensitivity to both types of dsHvCOPIs production. Ingestion of bacterially expressed dsHvαCOPI and dsHvγCOPI led to the highest mortality in both larvae and adults. Interestingly, there was a significant positive relationship between the knockdown efficiency of different HvCOPI subunits and the corresponding mortality on day 2 and 4, respectively. These combined results suggest that α and γ subunits of HvCOPI are promising targets for the development of RNAi-based biopesticides for the management of H. vigintioctopunctata.






Similar content being viewed by others
References
Bachman PM, Bolognesi R, Moar WJ et al (2013) Characterization of the spectrum of insecticidal activity of a double-stranded RNA with targeted activity against Western Corn Rootworm (Diabrotica virgifera virgifera LeConte). Transgenic Res 22:1207–1222
Baum JA, Bogaert T, Clinton W et al (2007) Control of coleopteran insect pests through RNA interference. Nat Biotechnol 25:1322–1326
Béthune J, Wieland FT (2018) Assembly of COPI and COPII vesicular coat proteins on membranes. Annu Rev Biophys 47:63–83
Burand JP, Hunter WB (2013) RNAi: future in insect management. J Invertebr Pathol 112:S68–S74
Fire A, Xu S, Montgomery MK, Kostas SA et al (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Chikami Y, Kawaguchi H, Suzuki T et al (2020) Oral RNAi of diap1 results in rapid reduction of damage to potatoes in Henosepilachna vigintioctopunctata. J Pest Sci. https://doi.org/10.1007/s10340-020-01276-w
Christiaens O, Whyard S, Vélez AM et al (2020) Double-stranded RNA technology to control insect pests: current status and challenges. Front Plant Sci 11:451
Guo Z, Kang S, Zhu X et al (2015) The novel ABC transporter ABCH1 is a potential target for RNAi-based insect pest control and resistance management. Sci Rep 5(1):13728
Haller S, Widmer F, Siegfried BD et al (2019) Responses of two ladybird beetle species (Coleoptera: Coccinellidae) to dietary RNAi. Pest Manag Sci 75:2652–2662
Jayaram SA, Senti KA, Tiklová K et al (2008) COPI vesicle transport is a common requirement for tube expansion in Drosophila. PLoS ONE 3(4):e1964
Liu FZ, Li X, Zhao MH et al (2020) Ultrabithorax is a key regulator for the dimorphism of wings, a main cause for the outbreak of planthoppers in rice. Natl Sci Rev nwaa061.
Liu FZ, Yang B, Zhang AH et al (2019) Plant-mediated RNAi for controlling Apolygus lucorum. Front Plant Sci 10:64
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 25:402–408
Lü J, Chen SM, Guo MJ et al (2018) Selection and validation of reference genes for RT-qPCR analysis of the ladybird beetle Henosepilachna vigintioctomaculata. Front Physiol 9:1614
Lü J, Guo W, Chen SM et al (2020a) Double-stranded RNAs targeting HvRPS18 and HvRPL13 reveal potential targets for pest management of the 28-spotted ladybeetle. Henosepilachna vigintioctopunctata Pest Manag Sci 76:2663–2673
Lü J, Liu ZQ, Guo W et al (2020b) Feeding delivery of dsHvSnf7 is a promising method for management of the pest Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Insects 11(1):34
Lü J, Liu ZQ, Guo W et al (2020c) Oral delivery of dsHvlwr is a feasible method for management of the pest Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Insect Sci. https://doi.org/10.1111/1744-7917.12784
Majidiani S, PourAbad RF, Laudani F et al (2019) RNAi in Tuta absoluta management: effects of injection and root delivery of dsRNAs. J Pest Sci 92:1409–1419
Mao J, Zhang P, Liu C et al (2015) Co-silence of the coatomer β and v-ATPase A genes by siRNA feeding reduces larval survival rate and weight gain of cotton bollworm, Helicoverpa armigera. Pestic Biochem Phys 118:71–76
Pan HP, Xu LH, Noland JE et al (2016) Assessment of potential risks of dietary RNAi to a soil micro-arthropod, Sinella curviseta Brook (Collembola: Entomobryidae). Front Plant Sci 7:1028
Pan HP, Yang XW, Bidne K et al (2017) Dietary risk assessment of v-ATPase A dsRNAs on monarch butterfly larvae. Front Plant Sci 8:242
Pan HP, Yang XW, Siegfried BD et al (2015) A comprehensive selection of reference genes for RT-qPCR analysis in a predatory lady beetle, Hippodamia convergens (Coleoptera: Coccinellidae). PLoS ONE 10:e0125868
Pan HP, Yang XW, Romeis J et al (2020) Dietary RNAi toxicity assay exhibits differential responses to ingested dsRNAs among lady beetles. Pest Manag Sci 76:3606–3614
Powell ME, Bradish HM, Gatehouse JA et al (2017) Systemic RNAi in the small hive beetle Aethina tumida Murray (Coleoptera: Nitidulidae), a serious pest of the European honey bee Apis mellifera. Pest Manag Sci 73:53–63
Prentice K, Christiaens O, Pertry I et al (2017) RNAi-based gene silencing through dsRNA injection or ingestion against the African sweet potato weevil Cylas puncticollis (Coleoptera: Brentidae). Pest Manag Sci 73:44–52
Roberts AF, Devos Y, Lemgo GN et al (2015) Biosafety research for non-target organism risk assessment of RNAi-based GE plants. Front Plant Sci 6:958
Rodrigues TB, Rieske LK, Duan JJ et al (2017) Development of RNAi method for screening candidate genes to control emerald ash borer. Agrilus planipennis Sci Rep 7(1):1–8
Shang F, Ding BY, Ye C et al (2020) Evaluation of a cuticle protein gene as a potential RNAi target in aphids. Pest Manag Sci 76:134–140
Sharma A, Thakur A, Kaur S et al (2012) Effect of Alternaria alternata on the coccinellid pest Henosepilachna vigintioctopunctata and its implications for biological pest management. J Pest Sci 85:513–518
Spang A, Matsuoka K, Hamamoto S et al (1998) Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. P Natl Acad Sci USA 95(19):11199–11204
Sparks TC, Nauen R (2015) IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121:122–128
Taning CNT, Christiaens O, Berkvens N et al (2016) Oral RNAi to control Drosophila suzukii: laboratory testing against larval and adult stages. J Pest Sci 89(3):803–814
Tu XY, Wang GH (2010) Research progress of bio-control of Henosepilachna vigintioctopunctata. Chinese Plant Prot 30:13–16
US EPA (2017) Human health and ecological risk assessments for SmartStax PRO (MON 89034 x TC1507 x MON 87411 x DAS-59122–7), a plant-incorporated protectant intended to control corn rootworm through ribonucleic acid (RNA) interference.
Vélez AM, Jurzenski J, Matz N et al (2016) Developing an in vivo toxicity assay for RNAi risk assessment in honey bees, Apis mellifera L. Chemosphere 144:1083–1090
Vélez AM, Fishilevich E (2018) The mysteries of insect RNAi: focus on dsRNA uptake and transport. Pestic Biochem Physiol 151:25–31
Vélez AM, Fishilevich E, Rangasamy M et al (2020) Control of western corn rootworm via RNAi traits in maize: lethal and sublethal effects of Sec23 dsRNA. Pest Manag Sci 76(4):1500–1512
Wang ZL, Li CR, Yuan JJ et al (2017) Demographic comparison of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae) reared on three cultivars of Solanum melongena L. and a wild host plant Solanum nigrum L. J Econ Entomol 110(5):2084–2091.
Yang X, Deng S, Wei X et al (2020) MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc Natl Acad Sci U S A pii: 201913603.
Zhang J, Khan SA, Hasse C et al (2015) Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 34:991–994
Zhang J, Khan SA, Heckel DG et al (2017) Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol 35:871–882
Zhou G, Isoe J, Day WA et al (2011) Alpha-COPI coatomer protein is required for rough endoplasmic reticulum whorl formation in mosquito midgut epithelial cells. PLoS ONE 6(3):e18150
Zhou X, Oi FM, Scharf ME (2006) Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc Natl Acad Sci U S A 103(12):4499–4504
Zhou X, Wheeler MM, Oi FM et al (2008) RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem Mol Biol 38(8):805–815
Zhu F, Xu J, Palli R et al (2011) Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag Sci 67:175–182
Zotti M, Dos Santos EA, Cagliari D et al (2018) RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag Sci 74:1239–1250
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31972269) and the National Key R&D Program of China (2017YFD0200900). The granting agencies have no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by G. Smagghe.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lü, J., Yang, C., Liu, Z. et al. Dietary RNAi toxicity assay suggests α and γ subunits of HvCOPI as novel molecular targets for Henosepilachna vigintioctopunctata, an emerging coccinellid pest. J Pest Sci 94, 1473–1486 (2021). https://doi.org/10.1007/s10340-021-01350-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01350-x