Abstract
Purpose
To evaluate the efficacy and safety of intravenous “freeze-dried sulfonated human normal immunoglobulin (GGS)” in patients with steroid-resistant optic neuritis (ON).
Study design
Multicenter, prospective, double-blind, parallel-group, randomized controlled trial.
Methods
Patients with steroid-resistant acute ON were randomly assigned to receive either intravenous GGS (GGS group) or intravenous methylprednisolone (steroid pulse [SP] group). Visual acuity (logarithm of the minimum angle of resolution [logMAR]), mean deviation (MD) value of the Humphrey Field Analyzer, and critical flicker fusion frequency were measured as efficacy endpoints; adverse events (AEs) were assessed as the safety endpoint.
Results
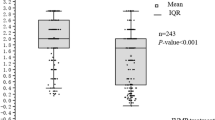
Thirty-two patients (16 patients/group) received the study drugs. The primary endpoint, change in logMAR at week 2 compared to baseline, showed no statistically significant intergroup difference. However, compared with the SP group, change in the GGS group was increasingly indicative of visual improvement, with least squares mean difference of > 0.3 logMAR. On post-hoc analyses, the percentage of patients in the GGS and SP groups with improvement by ≥ 0.3 logMAR at week 2 were 75.0% and 31.3%, respectively. Changes in MD values at week 2 compared to baseline were 9.258 ± 8.296 (mean ± standard deviation) dB and 3.175 ± 6.167 dB in the GGS and SP groups, respectively. These results showed statistically significant intergroup differences (visual acuity improvement, P = 0.032; change in MD values, P = 0.030). No clinically significant AEs were observed.
Conclusion
Our results suggest that intravenous immunoglobulin could be a safe and efficacious therapeutic option for prompt treatment of steroid-resistant acute ON. Trial registration: JapicCTI-132080.






Similar content being viewed by others
References
Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326:581–8.
Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol. 1993;111:773–5.
Wakakura M, Mashimo K, Oono S, Matsui Y, Tabuchi A, Kani K, et al. Multicenter clinical trial for evaluating methylprednisolone pulse treatment of idiopathic optic neuritis in Japan. Optic neuritis treatment trial multicenter cooperative research group (ONMRG). Jpn J Ophthalmol. 1999;43:133–8.
Kang H, Chen T, Li H, Xu Q, Cao S, Wei S. Prognostic factors and disease course in aquaporin-4 antibody-positive Chinese patients with acute optic neuritis. J Neurol. 2017;264:2130–40.
Kezuka T, Usui Y, Yamakawa N, Matsunaga Y, Matsuda R, Masuda M, et al. Relationship between NMO-antibody and anti-MOG antibody in optic neuritis. J Neuro-ophthalmol. 2012;32:107–10.
Bouzar M, Daoudi S, Hattab S, Bouzar AA, Deiva K, Wildemann B, et al. Neuromyelitis optica spectrum disorders with antibodies to myelin oligodendrocyte glycoprotein or aquaporin-4: clinical and paraclinical characteristics in Algerian patients. J Neurol Sci. 2017;381:240–4.
Jitprapaikulsan J, Chen JJ, Flanagan EP, Tobin WO, Fryer JP, Weinshenker BG, et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology. 2018;125:1628–37.
Ishikawa H, Kezuka T, Shikishima K, Yamagami A, Hiraoka M, Chuman H, et al. Epidemiologic and clinical characteristics of optic neuritis in Japan. Ophthalmology. 2019;126:1385–98.
Ruprecht K, Klinker E, Dintelmann T, Rieckmann P, Gold R. Plasma exchange for severe optic neuritis: treatment of 10 patients. Neurology. 2004;63:1081–3.
Roesner S, Appel R, Gbadamosi J, Martin R, Heesen C. Treatment of steroid-unresponsive optic neuritis with plasma exchange. Acta Neurol Scand. 2012;126:103–8.
Deschamps R, Gueguen A, Parquet N, Saheb S, Driss F, Mesnil M, et al. Plasma exchange response in 34 patients with severe optic neuritis. Neurology. 2016;263:883–7.
Nakao Y, Nakamura Y, Aomatsu K, Hirano M, Sakamoto H. Intravenous immunoglobulin treatment in corticosteroid refractory anti-aquaporin 4 antibody-seropositive optic neuritis. Neuro-ophthalmol Jpn. 2012;29:424–33 (in Japanese).
Tselis A, Perumal J, Caon C, Hreha S, Ching W, Din M, et al. Treatment of corticosteroid refractory optic neuritis in multiple sclerosis patients with intravenous immunoglobulin. Eur J Neurol. 2008;15:1163–7.
Altunrende B, Akdal G, Soylev-Bajin M, Yaman A, Kocaslan M, Nalbantoğlu M, et al. Intravenous immunoglobulin treatment for recurrent optic neuritis. Noro Psikiyatr Ars (Arch Neuropsychiatry). 2019;56:3–6.
Magraner MJ, Coret F, Casenova. The effect of intravenous immunoglobulin on neuromyelitis optica. Neurologia. 2013;28:65–72.
Viswanathan S, Wong AH, Quek AM, Yuki N. Intravenous immunoglobulin may reduce relapse frequency in neuromyelitis optica. J Neuroimmunol. 2015;282:92–6.
Wakakura M, Ishikawa S, Oono S, Tabuchi A, Kani K, Tazawa Y, et al. Incidence of acute idiopathic optic neuritis and its therapy in Japan. J Jpn Ophthalmol Soc. 1995;99:93–7 (in Japanese).
Nakao Y. New aspects of optic neuritis in multiple sclerosis and neuromyelitis optica. J Neuroimmunol. 2009;17:177–84 (in Japanese).
Matsumoto Y, Mori S, Ueda K, Kurimoto T, Kanamori A, Yamada Y, et al. Impact of the anti-aquaporin-4 autoantibody on inner retinal structure, function, and structure-function associations in Japanese patients with optic neuritis. PLoS ONE. 2017;12:e0171880.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. (International panel for NMO diagnosis): international consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89.
Mimura O, Fujikado T, Ueki S, Kezuka T, Shikishima K, Sugasawa J, et al. (Committee of guideline on diagnosis and management of anti-aquaporin-4 antibody-positive optic neuritis). Guideline on diagnosis and management of anti-aquaporin-4 antibody-positive optic neuritis. J Jpn Ophthalmol Soc. 2014;118:446–60 (in Japanese).
Havla J, Kümpfel T, Schinner R, Spadaro M, Schuh E, Meinl E, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol. 2017;264:139–51.
Peng A, Kinoshita M, Lai W, Tan A, Qiu X, Zhang L, et al. Retinal nerve fiber layer thickness in optic neuritis with MOG antibodies: a systematic review and meta-analysis. J Neuroimmunol. 2018;325:69–73.
Matsuda R, Kezuka T, Umazume A, Okunuki Y, Goto H, Tanaka K. Clinical profile of anti-myelin oligodendrocyte glycoprotein antibody seropositive cases of optic neuritis. Neuroophthalmology. 2015;39:213–9.
Kobayter L, Chetty S. Management of optic neuritis in Ireland: a survey comparing the management practices of acute demyelinating optic neuritis amongst ophthalmologists and neurologists in Ireland. Ir J Med Sci. 2019;188:277–82.
Das H, Gautam M, Lavaju P. An overview of idiopathic optic neuritis in eastern Nepal. Nepal J Ophthalmol. 2010;2:10–5.
Sun MH, Wang HS, Chen KJ, et al. Clinical characteristics of optic neuritis in Taiwanese children. Eye. 2011;25:1457–64.
Mori S, Kurimoto T, Ueda K, Nakamura M. Short-term effect of additional apheresis on visual acuity changes in patients with steroid-resistant optic neuritis in neuromyelitis optica spectrum disorders. Jpn J Ophthalmol. 2018;62:525–30.
Mimura O 2017 Diagnosis and management in neuro-ophthalmology, 2nd edition, IGAKU-SHOIN Ltd. Tokyo, pp. 64 (in Japanese).
Nomura K. The foundations and pitfall of intravenous immunoglobulin in treatment of neurological diseases. Neurological Therapeutics. 2014;31:183–7 (in Japanese).
Ratelade J, Smith AJ, Verkman AS. Human immunoglobulin G reduces the pathogenicity of aquaporin-4 autoantibodies in neuromyelitis optica. Exp Neurol. 2014;255:145–53.
Tradtrantip L, Felix CM, Spirig R, Morelli AB, Verkman AS. Recombinant IgG1 Fc hexamers block cytotoxicity and pathological changes in experimental in vitro and rat models of neuromyelitis optica. Neuropharmacology. 2018;133:345–53.
Nobuyoshi S, Kanamori A, Matsumoto Y, Nakamura M. Rescue effects of intravenous immunoglobulin on optic nerve degeneration in a rat model of neuromyelitis optica. Jpn J Ophthalmol. 2016;60:419–23.
Grünewald B, Bennett JL, Toyka KV, Sommer C, Geis C. Efficacy of polyvalent human immunoglobulins in an animal model of neuromyelitis optica evoked by intrathecal anti-aquaporin 4 antibodies. Int J Mol Sci. 2016;17:1407.
Takahashi H, Okuda S, Tamura M, Kamei S, Aizawa R, Kobayashi T. Prophylactic treatment with intravenous immunoglobulin attenuates experimental optic neuritis in mice. Biol Pharm Bull. 2019;42:173–8.
Schachat AP 2018 Ryan’s retina 6th ed. Elsevier, pp. 756.
Yamaguchi Y, Shirai K, Sumioka T, Takada Y, Iwanishi H, Okada Y, et al. A five-year review of optic neuritis in children at wakayama medical university hospital. Rinsho Ganka. 2019;73:341–6 (in Japanese).
Acknowledgements
Teijin Pharma Limited, the sponsor, provided funding for this study, determined the study design, and prepared the clinical study protocol. It also collected and analyzed the data and supported the writing of this report. The investigational and the control drugs used in this study were provided to Teijin Pharma Limited by Chemo-Sero-Therapeutic Research Institute (now, KM Biologics Co., Ltd.). We are deeply grateful to Dr. Yusaku Nakamura (Chairman, Department of Neurology, Sakai City Medical Center) for the central review of head/spinal cord MRI data. We also thank all investigators and the patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
O. Mimura, Grant (Teijin), Lecture fee (Santen, Senju, Otsuka, Pfizer, Alcon, GSK); H. Ishikawa, Grant (Teijin), Grant-in-aid for Scientific Research from Ministry of Health, Labor and Welfare 2015–2017 (Research Project on Intractable Diseases Policy); T. Kezuka, Grant, Financial support (Teijin), Financial support (AbbVie, Alcon, Cosmic Corp, Eisai, Kowa, Mitsubishi Tanabe, Santen, Senju), Non-Financial support (Cosmic Corp); K. Shikishima, Grant (Teijin), Honorarium for Speaker (Santen, Senju, Johnson & Johnson, Chugai, Cosmic Corp), T. Suzuki, Grant (Teijin); M. Nakamura, Grant (Teijin); H. Chuman, Grant (Teijin); K. Inoue, Grant (Teijin), Grant, Research support, Lecture fee, Writing assistance, Consultant fee (Santen), Research support (Alcon), Lecture fee (Otsuka), Grant, Research support, Lecture fee, Consultant fee (Senju), Lecture fee, Consultant fee (Kowa), Grant, Research support, Lecture fee (Allergan), Lecture fee (Novartis, Wakamoto), Research support (IQVIA, Mayo Corp, Lilly); A. Kimura, Grant (Teijin); A. Yamagami, Grant (Teijin); M. Mihoya, Employee (Teijin), Y. Nakao, Grant (Teijin).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Mimura, O., Ishikawa, H., Kezuka, T. et al. Intravenous immunoglobulin treatment for steroid-resistant optic neuritis: a multicenter, double-blind, randomized, controlled phase III study. Jpn J Ophthalmol 65, 122–132 (2021). https://doi.org/10.1007/s10384-020-00790-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00790-9