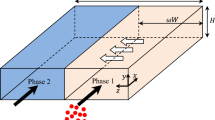
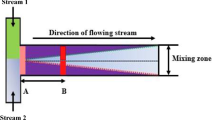
Abstract
An investigation of molecular diffusion of solutes across water/oil interfaces in a Y-Y-shaped microchannel with an integrated guide structure is presented. Finite volume numerical simulations were compared with experimental literature data. Analytical approaches including an infinite composite medium model, phase-specific mass transfer coefficient models, and a static transfer cell model were also assessed. An increase in accuracy for the mass transfer coefficient models was achieved by using local coefficients as opposed to length-averaged expressions. The static transfer cell model was shown to improve when based on the interfacial contact time, as opposed to the organic phase residence time. The results presented in this work have immediate application to the determination of kinetic rate constants in reactive mass transfer systems, as considered in Part II of this study (Ciceri et al. Microfluid Nanofluid, 2012).






Similar content being viewed by others
Abbreviations
- \(A_{\perp ,i}\) :
-
Cross-sectional area of microchannel half-volume (m\(^2\))
- \(A_{\rm ref} \) :
-
Total area of interface (m\(^2\))
- \({Bo}\) :
-
Bond number (\({Bo} = \Updelta \rho g D_{\rm h} ^2/\gamma \))
- \(c\) :
-
Local species concentration (mM)
- \({\tilde{c}}\) :
-
Spatially averaged species concentration (mM)
- \({\bar{c}}\) :
-
Velocity-averaged species concentration (mM)
- \({d}\) :
-
\(z\)-height of interface (m)
- \(D_{\rm h} \) :
-
Hydraulic diameter of microchannel half-volume (m)
- \({\fancyscript{D}} \) :
-
Diffusivity (m\(^2\)/s)
- \(F\) :
-
Volumetric flow rate (m\(^3\)/s)
- \(g\) :
-
Gravitational acceleration (m/s\(^2\))
- \(Gz\) :
-
Mass transfer Graetz number (\( Gz = Re \, Sc \, D_{\rm h} / x\))
- \(h_{\rm eq} \) :
-
Equivalent height (m)
- \(h_{\rm norm} \) :
-
Flow-rate-scaled equivalent height (m)
- \(I\) :
-
Identity tensor
- \(k_{i}\) :
-
Local mass transfer coefficient (m/s)
- \(\tilde{k}_{i}\) :
-
Average mass transfer coefficient (m/s)
- \(K_{\rm D} \) :
-
Distribution constant
- \(K_{\rm eff} \) :
-
Effective mass transfer coefficient (m/s)
- \(L\) :
-
Microchannel pass length (m)
- \(\hat{\bf {n}}\) :
-
Unit normal vector
- \({\rm MAPE}_{\rm exp} \) :
-
Mean Absolute Percentage Error (experimental reference)
- \({\rm MAPE}_{\rm sim} \) :
-
Mean Absolute Percentage Error (simulation reference)
- \(J_y\) :
-
Local diffusive flux of solute (mol/m\(^2\) s)
- \(\tilde{J}_y\) :
-
Average diffusive flux of solute (mol/m\(^2\) s)
- \(p\) :
-
Fluid pressure (Pa)
- \(R\) :
-
Interface radius of curvature (m)
- \(Re\) :
-
Reynolds number (\(Re=\rho \bar{u} D_{\rm h} /\upmu \))
- \(Sc\) :
-
Schmidt number (\(Sc = \upmu / \rho {\fancyscript{D}} \))
- \(Sh\) :
-
Sherwood number (\(Sh = k D_{\rm h} /{\fancyscript{D}} \))
- \(t\) :
-
Elapsed time of contact between phases (s)
- \(t^*\) :
-
Characteristic contact time scale (set either to \(t_{\rm org} \) or \(t_{\rm int} \)) (s)
- \(t_{\rm int} \) :
-
Interfacial contact time (\(t_{\rm int} = L/\bar{u}_{\rm int} \)) (s)
- \(t_{\rm org} \) :
-
Organic phase residence time (\(t_{\rm org} = V_{1/2} /F_{\rm org} \)) (s)
- \({\bf {u}} \) :
-
Fluid velocity (m/s)
- \(\bar{u}_{i}\) :
-
Mean fluid velocity (m/s)
- \(\bar{u}_{int}\) :
-
Mean interfacial velocity (m/s)
- \(W\) :
-
Microchannel \(y\)-half-width (m)
- \(V_{1/2}\) :
-
Microchannel half-volume (m\(^3\))
- \(x,y,z\) :
-
Cartesian coordinates (m)
- \(\tilde{y}\) :
-
Maximum \(y\)-deformation of the interface (m)
- \(\gamma \) :
-
Interfacial tension (N/m)
- \(\theta _{\rm max} \) :
-
Maximum interfacial contact angle (rad)
- \(\rho \) :
-
Fluid density (kg/m\(^3\))
- \(\tau \) :
-
Viscous stress tensor (Pa)
- \(\upmu \) :
-
Dynamic viscosity (Pa s)
- \(\phi \) :
-
Maximum angle subtended by the interfacial arc (rad)
- 0:
-
Initial
- aq:
-
Aqueous phase
- exp:
-
Experiment
- \(i\) :
-
\(i\)th phase
- inlet:
-
Pass-length inlet
- int:
-
Interface
- LM:
-
Logarithmic mean
- max:
-
Maximum
- \(n\) :
-
\(n\)th data point
- org:
-
Organic phase
- outlet:
-
Pass-length outlet
- sim:
-
Simulation
- 8HQ:
-
8-Hydroxyquinoline
- CFD:
-
Computational fluid dynamics
- DEHPA:
-
Di-(2-ethylhexyl) phosphoric acid
- LOC:
-
Lab-on-a-chip
- MAPE:
-
Mean Absolute Percentage Error
- TLM:
-
Thermal lens microscopy
- TRM:
-
Tetramethyl rhodamine
- STCM:
-
Static Transfer Cell Model
- SX:
-
Solvent extraction
- \(\upmu \)SX:
-
Microfluidic solvent extraction
References
Akpa BS, Matthews SM, Sederman AJ, Yunus K, Fisher AC, Johns ML, Gladden LF (2007) Study of miscible and immiscible flows in a microchannel using Magnetic Resonance Imaging. Anal Chem 79:6128–6134
Aota A, Hibara A, Kitamori T (2007) Pressure balance at the liquid-liquid interface of micro countercurrent flows in microchips. Anal Chem 79(10):3919–3924
Aota A, Mawatari K, Kitamori R (2009) Parallel multiphase microflows: fundamental physics, stabilization methods and applications. Lab Chip 9:2470–2476
Bird RB, Stewart WE, Lightfoot EN (2002) Transport Phenomena, 2nd edn, chap 2. John Wiley & Sons, Inc
Broboana D, Balan CM, Wohland T, Balan C (2011) Investigations of the unsteady diffusion process in microchannels. Chem Eng Sci 66(9):1962–1972
Ciceri D, Perera JM, Stevens GW (2011a) A study of molecular diffusion across a water/oil interface in a Y-Y shaped microfluidic device. Microfluid Nanofluid 11(5):593–600
Ciceri D, Perera JM, Stevens GW (2011b) Extraction of Co(II) by di (2-ethylhexyl) phosphoric acid in a microfluidic device. In: International Solvent Extraction Conference (ISEC 2011) Santiago, Chile
Ciceri D, Mason LR, Harvie DJE, Perera JM, Stevens GW (2012) Modelling of interfacial mass transfer in microfluidic solvent extraction: part II. Heterogeneous transport with reaction, Microfluid Nanofluid. doi:10.1007/s10404-012-1039-y
Crank J (1975) Diffusion in a plane sheet. In: The Mathematics of Diffusion, 2nd edn, chap 3. Clarendon Press, Oxford
Fick AE (1855) On liquid diffusion. Philos Mag 4(10):30–39
Frank-Kamenetskii DA (1969) Diffusion and Heat Transfer in Chemical Kinetics, 2nd edn. Plenum Press, New York
George PL, Borouchaki H (1998) Delaunay Triangulation and Meshing: Application to Finite Elements. Hermes
Geuzaine C, Remacle JF (2009) Gmsh: A 3-D finite element mesh generator with built-in pre- and post-processing facilities. Int J Numer Meth Eng 79(11):1309–1331
Harada M, Iwamoto K, Kitamori T, Sawada T (1993) Photothermal microscopy with excitation and probe beams coaxial under the microscope and its application to microparticle analysis. Anal Chem 65(20):2938–2940
Harvie DJE (2012) An implicit finite volume method for arbitrary transport equations. ANZIAM J 52:C1126–C1145
Higbie R (1935) The rate of absorption of a pure gas into a still liquid during a short time of exposure. Trans AIChE 31:365
Hotokezaka H, Tokeshi M, Harada M, Kitamori T, Ikeda Y (2005) Development of the innovative nuclide separation system for high-level radioactive waste using microchannel chip-extraction behavior of metal ions from aqueous phase to organic phase in microchannel. Prog Nucl Energ 47(1–4):439–447
Hotta T, Nii S, Yajima T, Kawaizumi F (2007) Mass tansfer characteristics of a microchannel device of split-flow type. Chem Eng Technol 30(2):208–213
Ismagilov RF, Stroock AD, Kenis PJA, Whitesides G, Stone HA (2000) Experimental and theoretical scaling laws for transverse diffusive broadening in two-phase laminar flows in microchannels. Applied Physics Letters 76(17):2376–2378
Kamholz AE, Yager P (2001) Theoretical analysis of molecular diffusion in pressure-driven laminar flow in microfluidic channels. Biophys 80(1):155–160
Kamholz AE, Weigl BH, Finlayson BA, Yager P (1999) Quantitative analysis of molecular interaction in a microfluidic channel: The T-sensor. Anal Chem 71:5340–5347
Kuban J, Dasgupta P, Berg PK (2003) Vertically stratified flows in microchannels. Computational simulations and applications to solvent extraction and ion exchange. Anal Chem 75(14):3549–3556
Lide DR (2009) CRC Handbook of Chemistry and Physics, 90th edn. CRC Press, Boca Raton
McCulloch JK, Kelly ED, Perera JM, White LR, Stevens GW, Grieser F (1996) Direct spectroscopic measurement and theoretical modeling of the diffusion of a single species in a two-phase unstirred system. J Colloid and Interf Sci 184(2):399–405
Minagawa T, Tokeshi M, Kitamori T (2001) Integration of a wet analysis system on a glass chip: Determination of Co (II) as 2-nitroso-1-napthol chelates by solvent extraction and Thermal Lens Microscopy. Lab Chip 1(1):72–75
Nadim A, Borhan A, Haj-Hariri H (1996) Tangential stress and Marangoni effects at a fluid-fluid interface in a Hele-Shaw cell. J Colloid Interf Sci 181:159–164
Nishi K, Perera JM, Misumi R, Kaminoyama M, Stevens GW (2010) Study of diffusion of Co(II) and Co(II)-DEHPA complex in a microfluidic device. J Chem Eng Jpn 43(4):342–348
Nishi K, Perera JM, Misumi R, Kaminoyama M, Stevens GW (2011) Flow and diffusion behaviour as a function of viscosity in a double-Y-type microfluidic device. J Chem Eng Jpn 44(7):509–517
Perry RH, Green DW (2007) Perry’s Chemical Engineers’ Handbook, 8th edn. McGraw-Hill, New York
Pohar A, Lakner M, Plazl I (2012) Parallel flow of immiscible liquids in a microreactor: modeling and experimental study. Microfluid Nanofluid 12:307–316
Sato K, Tokeshi M, Sawada T, Kitamori T (2000) Molecular transport between two phases in a microchannel. Anal Sci 16:455–456
Smirnova A, Mawatari K, Hibara A, Proskurnin MA, Kitamori T (2006) Micro-multiphase laminar flows for the extraction and detection of carbaryl derivative. Anal Chim Acta 558(1–2):69–74
Squires TM, Quake SR (2005) Microfluidics: Fluid physics at the nanoliter scale. Rev Mod Phys 77(3):977–1026
Surmeian M, Slyadnev MN, Hisamoto H, Hibara A, Uchiyama K, Kitamori T (2002) Three-layer flow membrane system on a microchip for investigation of molecular transport. Anal Chem 74(9):2014–2020
Tetala KKR, Swarts JW, Chen B, Janssen AEM, van Beek TA (2009) A three-phase microfluidic chip for rapid sample clean-up of alkaloids from plant extracts. Lab Chip 9(14):2085–2092
Tokeshi M, Minagawa T, Kitamori T (2000) Integration of a microextraction system on a glass chip: Ion-pair solvent extraction of Fe(II) with 4,7-diphenyl-1,10-phenanthrolinedisulfonic acid and tri-\(n\)-octylmethylammonium chloride. Anal Chem 72(7):1711–1714
Truskey GA, Yuan F, Katz DF (2004) Transport Phenomena in Biological Systems, 1st edn, chap 7. Prentice Hall
van Leeuwen M, Li X, Krommenhoek EE, Gardeniers H, Ottens M, van der Wielen LAM, Heijnen JJ, van Gulik WM (2009) Quantitative determination of glucose transfer between cocurrent laminar water streams in a H-shaped microchannel. Biotechnol Progr 25(6):1826–1832
van Male P, de Croon MHJM, Tiggelaar RM, van den Berg A, Schouten JC (2004) Heat and mass transfer in a square microchannel with asymmetric heating. Int J Heat Mass Tran 47(1):87–99
Whitesides G (2006) The origins and the future of microfluidics. Nature 442:368–373
Yaws CL (2003) Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds. Knovel
Žnidaršič-Plazl P, Plazl I (2007) Steroid extraction in a microchannel system—mathematical modelling and experiments. Lab Chip 7:883–889
Acknowledgments
Support from the Australian Research Council and the Particulate Fluids Processing Centre is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Mass transfer coefficient model
1.1 Derivation
The local flux \(J_y\) of solute in the \(y\)-direction is defined by
where \(k_{\rm {aq}}\) and \(k_{\rm {org}}\) are the local mass transfer coefficients, \(c_{i,{\rm {int}}}\) are the interfacial concentrations (per volume basis) and \(\bar{c}_i\) are the velocity-averaged solute concentrations given by
where \({A}_{\perp ,i}\) is the cross-sectional area of the relevant half of the channel. The distribution constant is defined, using Eq. 11, by
Substituting Eq. 46 into Eq. 44 gives
Eliminating \(c_{\rm aq,int }\) gives
For convenience, the following definitions are introduced:
so that
From the definition of \(J_y\) (Eq. 44),
Combining Eqs. 52 and 53 gives
Using the definition from Eq. 49 and taking the limit \({\rm d} A \rightarrow 0\) gives
Substituting Eq. 51 gives
Integrating this expression gives
where \(A_{\rm ref} \) is the total area of the interface. The total amount of solute transferred across the interface is given by
where \(\tilde{J}_y\) is the average flux of solute. Combining Eqs. 58 and 59 gives
1.2 Solution method
The aim is to solve for \(\bar{c}_{\rm org,outlet}\) for a fixed experimental \(\Updelta \bar{c}_ {\rm inlet}\). From mass conservation,
Substituting
into Eq. 63 gives
where, for constant values of \(\tilde{k}_i\) (‘averaged coefficient A & B’), \(\Updelta \bar{c}_{\rm outlet}\) is determined from Eq. 57,
Equation 66 is substituted into Eq. 65 in order to determine \(\bar{c}_{\rm aq,outlet}\). \(\bar{c}_{\rm org,outlet}\) can then be determined from Eq. 64.
For \(x\)-dependent \(k_i\) values (‘local coefficient A & B’), \(\Updelta \bar{c}_{\rm outlet} \) is determined using numerical integration:
where
and
Here, the substitution \({{\rm {d}}A} = d\,{\rm d}x\) has been made, where \(d\) is the \(z\)-height of the interface. The same solution method for determining \(\bar{c}_{\rm org,outlet}\) then follows as per the constant \(k_i\) case.
Appendix 2: Analytical expression for a heterogeneous thin slit
Building on the approach of Bird et al. (2002), an analytical expression was derived for two immiscible, incompressible liquids flowing in the \(y\)-direction of a thin slit of length \(L\) and effective width \(2D_{\rm h} \). The momentum flux for each phase \(i\) is given by the differential equation
where \(\Updelta p_i\) is the phase-specific pressure difference between the pass-length inlet and outlet. Integrating Eq. 70 for each phase gives
where \(\alpha _i\) is a constant of integration. The momentum flux across the interface (located at \(y=0\)) is assumed to be balanced:
Substituting Newton's law of viscosity and integrating gives
The solvent velocity was assumed to be continuous across the interface:
This gives a set of two equations to be solved simultaneously:
A no-slip boundary condition was applied on two fictional walls, each a distance \(D_{\rm h} \) from the centreline:
Solving for \(\alpha \) and \(\beta \) gives:
The average velocities \(\bar{u}_{x,i}\) are then given by:
Equations 78 and 79 are solved simultaneously for \(\Updelta p_i\). The interfacial velocity is then given by
Rights and permissions
About this article
Cite this article
Mason, L.R., Ciceri, D., Harvie, D.J.E. et al. Modelling of interfacial mass transfer in microfluidic solvent extraction: part I. Heterogenous transport . Microfluid Nanofluid 14, 197–212 (2013). https://doi.org/10.1007/s10404-012-1038-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1038-z