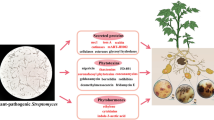
Abstract
Streptomyces species are best known for their ability to produce a wide array of medically and agriculturally important secondary metabolites. However, there is a growing number of species which, like Streptomyces scabies, can function as plant pathogens and cause scab disease on economically important crops such as potato. All of these species produce the phytotoxin thaxtomin, a nitrated dipeptide which inhibits cellulose synthesis in expanding plant tissue. The biosynthesis of thaxtomin involves conserved non-ribosomal peptide synthetases, P450 monooxygenases, and a nitric oxide synthase, the latter being required for nitration of the toxin. This nitric oxide synthase is also responsible for the production of diffusible nitric oxide by scab-causing streptomycetes at the host-pathogen interface, suggesting that nitric oxide production might play an additional role during the infection process. The thaxtomin biosynthetic genes are transcriptionally regulated by an AraC/XylS family regulator, TxtR, which is conserved in pathogenic streptomycetes and is encoded within the thaxtomin biosynthetic gene cluster. The TxtR protein specifically binds cellobiose, a known inducer of thaxtomin biosynthesis, and cellobiose is required for expression of the biosynthetic genes. A second virulence gene in pathogenic Streptomyces species, nec1, encodes a novel secreted protein that may suppress plant defence responses. The thaxtomin biosynthetic genes and nec1 are contained on a large mobilizable pathogenicity island; the transfer of this island to recipient streptomycetes likely explains the rapid emergence of new pathogenic species. The newly available genome sequence of S. scabies will provide further insight into the mechanisms utilized by pathogenic streptomycetes during plant-microbe interactions.







Similar content being viewed by others
References
Bérdy J (2005) Bioactive microbial metabolites. J Antibiot (Tokyo) 58:1–26
Bukhalid RA, Loria R (1997) Cloning and expression of a gene from Streptomyces scabies encoding a putative pathogenicity factor. J Bacteriol 179:7776–7783
Bukhalid RA, Takeuchi T, Labeda D, Loria R (2002) Horizontal transfer of the plant virulence gene, nec1, and flanking sequences among genetically distinct Streptomyces strains in the diastatochromogenes cluster. Appl Environ Microbiol 68:738–744
Clark CA, Matthews SW (1987) Histopathology of sweet potato root infection by Streptomyces ipomoea. Phytopathology 77:1418–1423
Deising HB, Werner S, Wernitz M (2000) The role of fungal appressoria in plant infection. Microbes Infect 2:1631–1641
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Fry BA, Loria R (2002) Thaxtomin A: evidence for a plant cell wall target. Physiol Mol Plant Pathol 60:1–8
Gartemann K-H, Kirchner O, Engemann J, Gräfen I, Eichenlaub R, Burger A (2003) Clavibacter michiganensis subsp. michiganensis: first steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J Biotechnol 106:179–191
Gusarov I, Nudler E (2005) NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria. Proc Natl Acad Sci USA 102:13855–13860
Healy FG, Wach M, Krasnoff SB, Gibson DM, Loria R. (2000) The txtAB genes of the plant pathogen Streptomyces acidiscabies encode a peptide synthetase required for phytotoxin thaxtomin A production and pathogenicity. Mol Microbiol 38:794–804
Healy FG, Krasnoff SB, Wach M, Gibson DM, Loria R (2002) Involvement of a cytochrome P450 monooxygenase in thaxtomin A biosynthesis by Streptomyces acidiscabies. J Bacteriol 184:2019–2029
Hodgson DA (2000) Primary metabolism and its control in streptomycetes: a most unusual group of bacteria. Adv Microb Physiol 42:47–238
Johnson EG, Joshi MV, Gibson DM, Loria R (2007a) Cello-oligosaccharides released from host plants induce pathogenicity in scab-causing Streptomyces species. Physiol Mol Plant Pathol. doi:10.1016/j.pmpp.2007.09.003
Johnson EG, Sparks JP, Dzikovski B, Crane BR, Gibson DM, Loria R (2007b) Plant-pathogenic Streptomyces species produce nitric oxide synthase-derived nitric oxide in response to host signals. Chem Biol 15(1):43–50
Joshi M, Rong X, Moll S, Kers J, Franco C, Loria R (2007a) Plant pathogenic Streptomyces secrete a novel virulence protein, Nec1, which facilitates infection. Mol Plant Microbe Interact 20:599–608
Joshi MV, Bignell DRD, Johnson EG, Sparks JP, Gibson DM, Loria R (2007b) The AraC/XylS regulator TxtR modulates thaxtomin biosynthesis and virulence in Streptomyces scabies. Mol Microbiol 66:633–642
Kers JA, Wach MJ, Krasnoff SB, Widom J, Cameron KD, Bukhalid RA, Gibson DM, Crane BR, Loria R (2004) Nitration of a peptide phytotoxin by bacterial nitric oxide synthase. Nature 429:79–82
Kers JA, Cameron KD, Joshi MV, Bukhalid RA, Morello JE, Wach MJ, Gibson DM, Loria R (2005) A large, mobile pathogenicity island confers plant pathogenicity on Streptomyces species. Mol Microbiol 55:1025–1033
King RR, Lawrence CH, Clark MC, Calhoun LA (1989) Isolation and characterization of phytotoxins associated with Streptomyces scabies. J Chem Soc Chem Commun 13:849–850
Kowalczewska M, Raoult D (2007) Advances in Tropheryma whipplei research: the rush to find biomarkers for Whipple’s disease. Future Microbiol 2:631–642
Lawrence CH, Clark MC, King RR (1990) Induction of common scab symptoms in aseptically cultured potato tubers by the vivotoxin, Thaxtomin. Phytopathology 80:606–608
Loria R, Kers J, Joshi M (2006) Evolution of plant pathogenicity in Streptomyces. Annu Rev Phytopathol 44:469–487
Möller M, Botti H, Batthyány C, Rubbo H, Radi R, Denicola A (2005) Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem 280:8850–8854
Monteiro-Vitorello CB, Camargo LE, Van Sluys MA et al. (2004) The genome sequence of the Gram-positive sugarcane pathogen Leifsonia xyli subsp. xyli. Mol Plant Microbe Interact 17:827–836
Muscatello G, Leadon DP, Klayt M, Ocampo-Sosa A, Lewis DA, Fogarty U, Buckley T, Gilkerson JR, Meijer WG, Vazquez-Boland JA (2007) Rhodococcus equi infection in foals: the science of ‘rattles’. Equine Vet J 39:470–478
Neill SJ, Desikan R, Hancock JT (2003). Nitric oxide signalling in plants. New Phytol 159:11–35
Rubbo H, Botti H, Batthyány C, Trostchansky A, Denicola A, Radi R (2002) Antioxidant and diffusion properties of nitric oxide in low-density lipoprotein. Meth Enzymol 359:200–209
Scheible W-R, Fry B, Kochevenko A, Schindelasch D, Zimmerli L, Somerville S, Loria R. Somerville CR (2003) An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 15:1781–1794
Sundaramurthy V, Pieters J (2007) Interactions of pathogenic mycobacteria with host macrophages. Microbes Infect 9(14–15):1671–1679
Trujillo ME, Goodfellow M (2003) Numerical phenetic classification of clinically significant aerobic sporoactinomycetes and related organisms. Antonie van Leeuwenhoek 84:39–68
Wach MJ, Kers JA, Krasnoff SB, Loria R, Gibson DM (2005) Nitric oxide synthase inhibitors and nitric oxide donors modulate the biosynthesis of thaxtomin A, a nitrated phytotoxin produced by Streptomyces spp. Nitric Oxide 12:46–53
Wach MJ, Krasnoff SB, Loria R, Gibson DM (2007) Effect of carbohydrates on the production of thaxtomin A by Streptomyces acidiscabies. Arch Microbiol 188:81–88
Wilson ID, Neill SJ, Hancock JT (2007) Nitric oxide synthesis and signalling in plants. Plant Cell Environ. doi:10.1111/j.1365-3040.2007.01761.x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loria, R., Bignell, D.R.D., Moll, S. et al. Thaxtomin biosynthesis: the path to plant pathogenicity in the genus Streptomyces . Antonie van Leeuwenhoek 94, 3–10 (2008). https://doi.org/10.1007/s10482-008-9240-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-008-9240-4