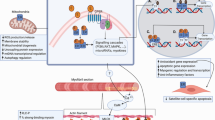
Abstract
Sarcopenia is a significant public health and medical concern confronting the elderly. Considerable research is being directed to identify ways in which the onset and severity of sarcopenia may be delayed/minimized. This paper provides a detailed identification and assessment of hormetic dose responses in animal model muscle stem cells, with particular emphasis on cell proliferation, differentiation, and enhancing resilience to inflammatory stresses and how this information may be useful in preventing sarcopenia. Hormetic dose responses were observed following administration of a broad range of agents, including dietary supplements (e.g., resveratrol), pharmaceuticals (e.g., dexamethasone), endogenous ligands (e.g., tumor necrosis factor α), environmental contaminants (e.g., cadmium) and physical agents (e.g., low level laser). The paper assesses both putative mechanisms of hormetic responses in muscle stem cells, and potential therapeutic implications and application(s) of hormetic frameworks for slowing muscle loss and reduced functionality during the aging process.

Modified from: Calabrese and Baldwin (1998)

Modified from: Chen et al. (2019) *P ≤ 0.05

Modified from: Hosseini et al. (2016) *P ≤ 0.05

Modified from Wang et al. (2016a) *P ≤ 0.05

Modified from: Bosutti and Degens (2015) *P ≤ 0.05

Modified from: Wang et al. (2016b) *P ≤ 0.05

Modified from: Orzechowski et al. (2002b) *P ≤ 0.05

Modified from: Orzechowski et al. (2002b) *P ≤ 0.05

Modified from: Orzechowski et al. (2002b) *P ≤ 0.05

Modified from: Orzechowski et al. (2002b) *P ≤ 0.05

Modified from: Caporossi et al. (2003)

Modified from: Soltow et al. (2010) *P ≤ 0.05

Modified from: Chen et al. (2007) *P ≤ 0.05

Modified from: Glass et al. (2011) *P ≤ 0.05

Modified from: Glass et al. (2011) *P ≤ 0.05

Modified from: Xu et al. (2008) *P ≤ 0.05

Modified from: Salgarella et al. (2017) *P ≤ 0.05

Modified from: Yano and Marcondes (2005) *P ≤ 0.05
Similar content being viewed by others
References
Abe MK, Chao TS, Solway J, Rosner MR, Hershenson MB (1994) Hydrogen peroxide stimulates mitogen-activated protein kinase in bovine tracheal myocytes: implications for human airway disease. Am J Respir Cell Mol Biol 11:577–585
Abreu P, Serna JDC, Munhoz AC, Kowaltowski AJ (2020) Calorie restriction changes muscle satellite cell proliferation in a manner independent of metabolic modulation. Mech Age Dev 192:111362
Abrunhosa VM, Soares CP, Possidonio ACB, Alvarenga AV, Costa-Felix RP, Costa ML (2014) Induction of skeletal muscle differentiation in vitro by therapeutic ultrasound. Ultrasound Med Biol 40:504–512
Alvarez B, Quinn LS, Busquets S, Quiles MT, Lopez-Soriano FJ, Argiles JM (2002) Tumor necrosis factor-α exerts interleukin-6-dependent and -independent effects on cultured skeletal muscle cells. Biochim Biophys Acta 1542:66–72
Alway SE, Myers MJ, Mohamed JS (2014) Regulation of satellite cell function on sarcopenia. Aging Neurosci 6:246
Anderson JE (2000) A role for nitric oxide in muscle repair: Nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell 11(5):1859–1874
Assis L, Moretti AI, Abrahao TB, de Souza HP, Hamblin MR, Parizotto NA (2013) Low-level laser theapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci 28:947–955
Attele AS, Zhou YP, Xie JT, We JA, Zhang I, Dey I, Pugh W, Rue PA, Polonsky KS, Yuan CS (2002) Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 51:1851–1858
Bae YS, Oh H, Rhee SG, Yoo YD (2011) Regulation of reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589:2189–2138
Bareja A, Draper JA, Katz LH, Lee DE, Grimsrud PA, White JP (2021) Chronic caloric restriction maintains a youthful phosphoproteome in aged skeletal muscle. Mech Age Dev 195:111443
Betters JL, Lira VA, Drenning JA, Soltow QA, Criswell DS (2008) Supplemental nitric oxide augments satellite cell activity on cultured modifiers from aged mice. Exp Gerontol 43:10941101
Bosutti A, Degens H (2015) The impact of resveratrol and hydrogen peroxide a muscle cell plasticity shows a dose-dependent interaction. Sci Rep 5:8093
Brown JD, Hancock CR, Mongillo AD, Baron JB, Digiovanni RA, Parcell AC, Winder WW, Thomson DM (2011) Effect of LKB1 deficiency on mitochondrial content, fiber type, and muscle performance in the mouse diaphragm. Acta Physiol 201:457–466
Brunnelli RM, Rodrigues NC, Ribeiro DA, Fernandes K, Magri A, Assis L, Parizotto NA, Cliquet A Jr, Renno AC, Abreu DC (2014) The effects of 780-nm low level laser therapy on muscle healing process after cryolesion. Lasers Med Sci 19:91–96
Calabrese EJ (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 27:1451–1474
Calabrese EJ (2011) Toxicology rewrites its history and rethinks its future: giving equal focus to both harmful and beneficial effects. Environ Toxicol Chem 30:2658–2673
Calabrese EJ (2013) Hormetic mechanisms. Crit Rev Toxicol 43:580–606
Calabrese EJ (2014) The dose-response: a fundamental concept in toxicology. In: Hayes AW, Kruger CL (eds) Principles and methods of toxicology, 6th edn. CRC Press, Boca Raton, pp 89–140
Calabrese EJ (2016a) Preconditioning is hormesis Part I. Pharm Res 110:265–275
Calabrese EJ (2016b) Preconditioning is hormesis part II: How the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharm Res 110:265–275
Calabrese EJ (2021a) Hormesis and adult adipose-derived stem cells. Pharm Res 172:105802
Calabrese EJ (2021b) Hormesis and apical papilla stem cells. Chem-Biol Interact
Calabrese EJ (2021c) Hormesis and endothelial stem cells. Dose Res 20(1):15593258211068625
Calabrese EJ (2021d) Hormesis and Bone Marrow Stem Cells: enhancing cell proliferation, differentiation and resilience to inflammatory stress. Chem-Biol Inter 351:109730
Calabrese EJ (2021e) Human periodontal ligament stem cells and hormesis: Enhancing cell renewal and cell differentiation. Pharm Res 173:105914
Calabrese EJ (2021f) Induced pluripotent stem cells and hormesis. Dose Res 2022:1–9
Calabrese EJ (2021g) Hormesis mediated acquired resilience: using plant-derived chemical to enhance health. Ann Rev Food Sci Tech 12:355–381
Calabrese EJ (2022) Hormesis—mouse embryonic stem cells. Chem Biol Inter 352:109783
Calabrese EJ, Baldwin LA (1998) A general classification of U-shaped dose-response relationships in toxicology and their mechanistic foundations. Hum Exper Toxicol 17:353–364
Calabrese EJ, Baldwin L (2000a) Chemical hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol 19:2–31
Calabrese EJ, Baldwin LA (2000b) The marginalization of hormesis. Hum Exp Toxicol 19:32–40
Calabrese EJ, Baldwin LA (2000c) Its historical foundations as a biological hypothesis. Hum Exp Toxicol 19:41–75
Calabrese EJ, Baldwin LA (2000d) Radiation hormesis: the demise of a legitimate hypothesis. Hum Exp Toxicol 19:76–84
Calabrese EJ, Baldwin LA (2000e) Tales of two similar hypotheses: the rise and fall of chemical and radiation hormesis. Hum Exp Toxicol 19:85–97
Calabrese EJ, Baldwin LA (2002) Defining hormesis. Hum Exp Toxicol 21:91–97
Calabrese EJ, Blain RB (2011a) The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Reg Toxicol Pharm 61:73–81
Calabrese EJ, Mattson MP (2011b) Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal 5:25–38
Calabrese EJ, Mattson MP (2017) How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis 3:13
Calabrese EJ, Agathoeous E, Kapoor R, Dhawan G, Calabrese V, Giordano J (2021a) Hormesis and neural stem cells. Free Radic Biol Med 178L314–329
Calabrese EJ, Agathokleous E, Dhawan G, Kapoor R, Calabrese V (2021b) Human dental pulp cells and hormesis. Aging Res Rev 101540
Caporossi D, Ciafre SA, Pittaluga M, Savini I, Farace MG (2003) Cellular responses to H2O2 and bleomycin-induced oxidative stress in L6C5 rat myoblasts. Free Radic Biol Med 35(11):1355–1364
Cerletti M, Jang YC, Finley LWS, Haigis MC, Wagers AJ (2012) Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell 10:515–519
Chakravarthy MV, Booth FW, Spangenburg EE (2001) The molecular responses of skeletal muscle satellite cells to continuous expression of IGF-1: implications for the rescue of induced muscular atrophy in aged rats. Int J Sport Nutr Exerc Metab 11(Suppl):S44–S48
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Chen SE, Gerkern E, Zhang Y, Mohan RK, Li AS, Reid MB, Li YP (2005) Role of TNF-α signaling in regeneration of cardiotoxin-injured muscle. Am J Physiol-Cell Physiol 289:C1179–C1187
Chen S-E, Jin B, Li Y-P (2007) TNF-α regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol-Cell Physiol 292(5):C1660–C1671
Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W (2009) Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FaxO1 pathway. Biochem Biophys Res Commun 378:389–393
Chen X, Guo Y, Jia G, Zhao H, Liu G, Huang Z (2019) Ferulic acid regulates muscle fiber type formation through the Sirt1/AMPK signaling pathway. Food Funct 10:259–265
Christman MF, Morgan RW, Jacobson FS, Ames BN (1985) Positive control of a regulon for a defense against oxidative stress and heat shock proteins in Salmonella typhimurium. Cell 41:753–762
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Gorospe M, de Cabo R, Sinclair DA (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392
Coolican SA, Samuel DS, Ewton DZ, McWasde FJ, Florin JR (1997) The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272:6653–6662
Davies KJA (1999) The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. UBMB Life 48:41–47
Davies JMS, Lowry CV, Davies KJA (1995) Transient adaptation to oxidative stresss in yeasts. Arch Biochem Biophys 317:1–6
Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop Relat Res 355:57–521
Faulkner JA, Brooks SV, Zerba E (1995) Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci 50:124–129
Ferraresi O, Kalppert B, Avci P, Huang Y-Y, de Sousa MVP, Bagnato VS, Parizotto NA, Hamblin MR (2015) Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochem Photobiol 91:411–416
Fisher BD, Hiller CM, Rennie SG (2003) A comparison of continuous ultra-sound and pulsed ultrasound on soft tissue injury markers in the rat. J Phys Ther Sci 15:65–70
Gaubin Y, Vaissade R, Croute F, Beau B, Soleihavoup J-P, Murat J-C (2000) Implications of free radicals and glutathione in the mechanisms of calcium-induced expression of stress proteins in the A549 human lung cell-line. Biochim Biophys Act 1495:4–13
Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC (2003) Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol 163(4):1417–1428
Ghosh S, Lertwattanarak R, Garduno JD, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N (2015) Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol Series A 70(2):232–246
Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J (2011) TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. PNAS 108:1585–1590
Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr (2000) NF -kappa B-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289:2363–2366
Harry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J (2008) Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res 26:1238–1244
Harry LE, Sandison A, Pearse MF, Paleoolog EM, Nanchahal J (2009) Comparison of the vascularity of fasciocutaneous tissue and muscle for coverage of open tibial fractures. Plast Reconstr Surg 124:1211–1219
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Herbert JM, Bono F, Savi P (1996) The mitogenic effect of H2O2 for vascular smooth muscle cells is mediated by an increase of the affinity of basic fibroblast growth factor for its receptor. FEBS Lett 395:43–47
Hosseini SA, Tabandeh MR, Namin SAM (2016) Promoting effect of ginsenoside Rb1 for GLUT-4 gene expression and cellular synthesis in C2Cl2 muscle cells. Intern J Pharm Res All Sci 5(2):151–158
Ikeda K, Takayama T, Suzuki N, Shimada K, Otsuka K, Ito K (2006) Effects of low-intensity pulsed ultrasound on the differentiation of C2Cl2 cells. Life Sci 79:1936–1943
Jang YC, Sinha M, Cerletti M, Dall’Osso C, Wagers AJ (2011) Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb Symp Quant Biol 76:101–111
Jackson MJ (2008) Redox regulation of skeletal muscle. IUBMB 60:497–501
Jariwalla RJ (2001) Rice-bran products: phytonutrients with potential applications in preventive and clinical medicine. Drugs Exp Clin Res 27:17–26
Joanisse S, Sniders T, Nederveen JP, Parise G (2018) The impact of aerobic exercise on the muscle stem cell response. Exer Sport Sci Rev 46(5):180–187
Kumar A, Murphy R, Robinson P, Wei L, Boriek SM (2004) Cyclic mechanical stain inhibits skeletal myogenesis through activation of focal adhesion kinase., Rac-1 GTPase, and NF-kB transcription factor. Faseb J 18:1524–1535
Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM (2010) Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 33:1497–1499
Lee KH, Kim DG, Shin NY, Song WK, Kwon H, Chung CH, Kang MS (1997) NF-kappa B-dependent expression of nitric oxide synthase is required for membrane fusion of chick embryonic myoblasts. Biochem J 324:237–242
Li Y-P (2003) TNF-α is a mitogen in skeletal muscle. Am J Physiol Cell Physiol 285:C370–C376
Lim JW, Kim H, Kim KH (2001) Nuclear factor kappa b regulates cyclooxygenase-2-expression and cell proliferation in human gastric cancer cells. Lab Invest 81:349–360
Liu L, Wang P, Liu X, He D, Liang C, Yu Y (2014) Exogenous NAD+ supplementation protects H9c2 cardiac myoblasts against hypoxia/re-oxygenation injury via Sirt1-p53 pathway. Fund Clin Pharm 28:180–189
Long JH, Lira VA, Soltow QA, Bettes JL, Sellman JE, Criswell DS (2006) Arginine supplementation induces myoblast fusion via augmentation of nitric oxide production. J Muscle Res Cell Motil 27:577–584
Lopez-Martins RAB, Marcos RL, Leonardo PS, Prianti AC, Muscara MN, Aimbire F, Frigo L, Iversen VV, Bjordal JM (2006) Effect of low-level laser (Ga-Al-As655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol 101:283–288
Masoro EJ (1998) Hormesis and the antiaging action of dietary restriction. Exper Gerontol 33:61–66
Mathew S, Abraham TE (2004) Ferulic acid: an antioxidant found naturally in plant cell walls and feruoyl esterases involved in its release and their applications. Crit Rev Biotech 24:59–83
Mehlen P, Hickey E, Weber LA, Arigo AP (1997) Large unphosphorylated aggregates as the active form of Hsp27 which controls intracellular reactive oxygen species and gluthathione levels and generates a protection against TNF-α in NIH-3T3 ras cells. Biochim Biophys Res Commun 241:187–192
Mesquita-Ferrari RA, Alves AN, de Oliveira CV, Artiheiro PP, Bussadori SK, Rocha LA, Nunes FD, Fernandes KPS (2015) Low-level laser irradiation modulates cell viability and creatine kinase activity in C2Cl2 muscle cells during the differentiation process. Lasers Med Sci 30:2209–2213
Mattson MP (2008) Hormesis defined age. Res Rev 8:1–7
Mcleod IA (2004) Low-level laser therapy in athletic training. Hum Kinet-ATT 9:17–21
McKibbin B (1978) The biology of fracture healing in long bones. J Bone Joint Surg Br 60:150–162
Muralidhara VLK (2014) Ameliorative effects of ferulic acid against lead acetate-induced oxidative stress, mitochondrial dysfunctions and toxicity in prepubertal rat brain. Neurochem Res 39:2501–2515
Orzechowski A, Grizard J, Jank M, Gajkowska B, Lokociejewska M, Zaron-Teperek M, Godlewski M (2002a) Dexamethasone-mediated regulation of death and differentiation of muscle cells. Is hydrogen peroxide involved in the process? Reprod Nutr Dev 42:197–216
Orzechowski A, Lokociejewska M, Muras P, Hocquette J-F (2002b) Preconditioning with millimolar concentrations of vitamin C or N-acetylcysteine protects L6 muscle cells insulin-stimulated viability and DNA synthesis under oxidative stress. Life Sci 71:1793–1808
Pan Y, Xu XY, Liu TC, Zhao XF, Zhang Y, Wen L (2006) Change in the generation of reactive oxygen species in electrical-stimulated C2C12. China J Sports Med 25:46–49
Pisconti A, Brunelli S, Di Padova M, De Palma C, Deponti D, Baesso S, Sartonerri V, Cossu G, Clementi E (2006) Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol 172:233–244
Price NI, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BL, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690
Reid MB (2001) Redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol 90:724–731
Salgarella AR, Cafarelli A, Ricotti L, Capineri L, Dario P, Menciassi A (2017) Optimal ultrasound exposure conditions for maximizing C2Cl2 muscle cell proliferation and differentiation. Ultrasound Med Biol 43(7):1452–1465
Sadanaga-Akiyoshi P, Yao H, Tanuma SI, Nakahara T, Hong JS, Ibayashi S, Fujishima M (2003) Nicotinamide attenuates focal ischemic brain injury in rats: with special reference to changes in nicotinamide and NAD+ levels in ischemic core and penumbra. Neurochem Res 28:1227
Seidki A, Lekouch N, Gamon S, Pineau A (2003) Toxic and essential trace metals in muscle, liver and kidney of bovines from a polluted area of Morocco. Sci Total Environ 317:201–205
Shefer G, Partridge TA, Heslop L, Gross JG, Uri O, Orna H (2002) Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci 115:1461–1469
Snijders T, Verdijk LB, van Loon LJ (2009) The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Age Res Rev 8:328–338
Soltow QA, Lira VA, Betters JL, Long JHD, Sellman JE, Zeanah EH, Criswell DS (2010) Nitric oxide regulates stretch-induced proliferation in C2Cl2 myoblasts. J Muscle Res Cell Motil 31:215–225
Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L (2008) Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 11:693–700
Tatsumi R, Hattori A, Ikeuchi Y, Andersn JE, Allen RE (2002) Release of hepatocyte growth factor from mecaicall stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Bio Cell 13:290a–2918
Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE (2006) Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol 190(6):C1487–C1494
Tinetti ME (2001) Where is the vision for fall prevention? J Am Geriatr Soc 49:676–677
Tong DL, Zhang DX, Xiang F, Teng M, Jing XP, Hou JM, Huang YS (2012) Nicotinamide pretreatment protects cardiomyocytes against hypoxia-induced cell death by improving mitochondrial stress. Pharmacology 90:11–18
Torrente Y, El Fahime E, Caron NJ, Del Bo R, Belicchi M, Pisati F, Tremblay JP, Bresolin N (2003) Tumor necrosis factor-α (TNF-α) stimulates chemotactic response in mouse myogenic cells. Cell Transp 12:91–100
Trajano LASN, Sergio LPS, Silva CL, Carvalho L, Al M, Stumbo AC, Fonseca AS (2016a) Low-level laser irradiation alters mRNA expression from genes involved in DNA repair and genomic stabilization in myoblasts. Laser Phys Lett 13:075601
Trajano LASN, Stumbo AC, Da Silva CL, Al M, Fonscea AS (2016b) Low-level infrared laser modulates muscle repair and chromosome stabilization genes in myoblasts. Lasers Med Sci 31:1161–1167
Vandenburgh HH, Shansky J, Karlisch P, Solerissi RL (1993) Mechanical stimulation of skeletal muscle generates lipid related second messengers by phospholipase activation. J Cell Physiol 155:63–71
Wang YX, Dumont N, Rudnicki MA (2014) Muscle stem cells at a glance. J Cell Sci 127:4543–4548
Wang H, Liang X, Luo G, Ding M, Liang Q (2016a) Protection effect of nicotinamide on cardiomyoblast hypoxia/re-oxygenation injury: study of cellular mitochondrial metabolism. Mol BioSyst 12:2257–2264
Wang L, Zhang T, Xi Y, Yang C, Sun C, Li D (2016b) Sirtuin 1 promotes the proliferation of C2C12 myoblast cells via the myostatin signaling pathway. Mol Med Rep 14:1309–1315
Wang L, Xue R, Sun C, Yang C, Xi Y, Zhang F, He Q, Wang S, Zhao F, Zhang Y, Li D (2016c) SIRT1 regulates C2C12 myoblast cell proliferation by activating Wnt signaling pathway. Int J Clin Exp Pathol 9(3):2857–2868
Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP (2002) Physiological role of tumor necrosis factor a in traumatic muscle injury. FASEB J 16:1630–1632
Wehrle U, Sterho FT, Du S, Pete D (1994) Effects of chronic electrical stimulation on myosin heavy chain expression in satellite cell cultures derived from rat muscles of different fiber-type composition. Differentiation 58:37–46
Wiess AG, Pacifici RE, Davies KJA (1995) Transient adaptation to oxidative stress in mammalian cells. Arch Biochem Biophys 318:231–240
Xu X-Y, Liu TC-Y, Duan R, Liu X-G (2003) The effects of low-intensity laser irradiation on the fatigue induced by dysfunction of mitochondria. In: Proc of SPIE od Third international conference on photonics and imaging in biology and medicine. Wuhan, China.
Xu X, Zhao X, Liu TC-Y, Pan H (2008) Low-intensity laser irradiation improves the mitochondrial dysfunction of C2Cl2 induced by electrical stimulation. Photomed Laser Surg 26(3):197–202
Yaffe D, Saxel O (1977) Serial passing and differentiation of myogenic cells isolated from dystropic mouse muscle. Nature 270:725–727
Yang JL, Chao JL, Lin JG (1996) Reactive oxygen species may participate in mutagenicity and mutational spectrum of cadmium in Chinese hamster oveary-K1 cells. Chem Res Toxicol 9:1360–1367
Yang ZF, Yang JG, Gao GH, Hu ZR, Chen HX, Qian HW (2002) Mechanism of bio-effects of low intensity laser radiation. Acta Laser Biol Sinica 11:388–394
Yano CL, Marcondes MCCG (2005) Cadmium chloride-induced oxidative stress in skeletal muscle cells in vitro. Free Rad Biol Med 39:1378–1384
Yun SN, Moon SJ, Ko SK, Bo Im, Chung SH (2004) Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch Pharm Res 27:790–796
Zalin RJ (1987) The role of hormones and prostanoids in the in vitro proliferation and differentiation of human myoblasts. Exp Cell Res 172(2):265–281
Zhao X, Pathak JL, Huang W, Zhu C, Li Y, Guan H, Zeng S, Ge L, Shu Y (2020) Metformin enhances osteogenic differentiation of stem cells from human exfoliated deciduous teeth through AMPK pathway. J Tissue Eng Regen Med 14:1869–1879
Acknowledgements
EJC acknowledges longtime support from the US Air Force (AFOSR FA9550-19-1-0413) and ExxonMobil Foundation (S18200000000256). The U.S. Government is authorized to reproduce and distribute for governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the author and should not be interpreted as necessarily representing policies or endorsement, either expressed or implied. Sponsors had no involvement in study design, collection, analysis, interpretation, writing and decision to and where to submit for publication consideration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Calabrese, E.J., Calabrese, V. Enhancing health span: muscle stem cells and hormesis. Biogerontology 23, 151–167 (2022). https://doi.org/10.1007/s10522-022-09949-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-022-09949-y