Abstract
Purpose
In the wake of SARS-CoV-2’s global spread, human activities from health to social life to education have been affected. Favipiravir and Molnupiravir exhibited novel hexokinase inhibition and we discuss advantages of this property in their COVID-19 inhibition potential.
Methods
This paper describes molecular docking data of human hexokinase II with Favipiravir, Cyan 20, Remdesivir, 2DG, and Molnupiravir along with hexokinase inhibition assays.
Results
Favipiravir, an antiviral drug previously cleared for treating the flu and ebola, has shown some promise in early trials to treat COVID-19. We observed potent human hexokinase inhibiting potential of Favipiravir (50%) as against 4% and merely 0.3% hexokinase inhibition with Molnupiravir and 2 Deoxy d glucose at 0.1 mM concentration supported by molecular docking studies.
Conclusion
Favipiravir could continue to be part of the COVID-19 treatment regimen due to its resistance to host esterases, hexokinase inhibition potential and proven safety through human trials.
Similar content being viewed by others
Introduction
As COVID-19 specific treatment is not yet available, antiviral medications are being used as experimental adjuncts to supportive care for COVID-19-affected people (Nicola et al. 2020). The total number of confirmed global COVID-19 cases as of March 2022, is 468 million and more than 6 million deaths reported globally. After being declared a global pandemic by the World Health Organization (WHO), COVID-19 has resulted in the loss of livelihoods with a rippling effect on the global economy since March 2020 (Shang et al. 2021a). Even with attempts to contain the virus through measures such as masks, hand sanitizers, social distance and travel restrictions, and minimal social gatherings, transmission of the virus continues (Sanders et al. 2020; Abuga and Nyamweya 2021; Prajapati et al. 2022).
Although, measures like quarantine along with isolation have shown to be most effective measures of spread of COVID-19 transmission (Girum et al. 2020), treating viral infections using drugs like recombinant interferons (Darazam et al. 2021), viral polymerase inhibitors, both chemical (Chien et al. 2020) and herbal constituents (Saha et al. 2021), inhibitors of viral proteases (Sagawa et al. 2020; Su et al. 2020; Seth et al. 2020; Mengist et al. 2021; Forrestall et al. 2021) are reported. Inhibitors of mammalian host cell proteases such as ACE2 (Terali et al. 2020) that mediate entry of the virus into the target cell, endosomal inhibitors of viral deproteinization (Shang et al. 2021b) and drug preparations based on antiviral antibodies (Takashita et al. 2022) and hexokinase inhibitors (Mantha et al. 2021; Icard et al. 2021) are also effective. Favipiravir and Molnupiravir are viral protease inhibitors while 2DG affects infection-induced cytopathic effect through inhibition of cellular glycolysis (Sahu and Kumar 2021; Vangeel et al. 2022). Bhatt et al. (2022) in a recent finding, suggest that 2-DG can be used as a treatment regimen for COVID-19 since it effectively inhibits the SARS-CoV-2 multiplication and it weakens the potential of the progeny virus for further infection since glycolysis is a crucial step for replication of SARS-CoV-2 (Ajaz et al. 2021; Shen and Wang 2021).
An interesting correlation between hexokinase II expression and viral infection is discussed by Ramière et al. (2014) wherein a direct interaction between HCV NS5A protein and cellular HK2 is reflected due to increase in HK2 activity during HCV infection. Similarly, pharmacological inhibition of HK2 activity is reported to protect cells against hypertonic stress-induced apoptosis, reduces viral induced cell death, and reduce viral multiplication of human rhinovirus infection (HRV) (Courteau et al. 2015), newcastle disease virus (Al-Ziaydi et al. 2020) etc. Hence, an inhibitor of hexokinase 2 appears a potent drug for reducing viral load.
We accidentally observed strong interactions of Favipiravir with Human HK II and found it very relevant and crucial in the context of controlling COVID-19. To examine its true hexokinase inhibiting potential, two other drugs, namely Molnupiravir, and 2 DG have also been studied on similar lines and results are disclosed in the current article.
Materials and methods
Reagents and kits
Hexokinase calorimetric assay kit (Product no: MAK91) and 2-Deoxy-d-glucose were also purchased from Sigma Aldrich (USA). Favipiravir was procured from Honour Labs Limited, Telangana, India while Molnupiravir was supplied manufactured by Inchem Labs Pvt. Limited, Andhra Pradesh, India. Rest of the reagents used was of analytical grade, unless mentioned otherwise. The concentrations of the drugs used for the in-vitro assays were assayed by HPLC methods developed and validated in-house in order to ascertain their purity. The % purity of the compounds (between 99 and 100%) matched the CoA’s of the respective compounds shared by the supplier.
Hexokinase assay
The hexokinase (HK) assay was carried out using the HK calorimetric assay kit (Sigma, St. Louis, USA) as per manufacturer’s instructions. Briefly, from the inhibitor stock solution, a known amount of aliquot was withdrawn in order to achieve 0.1 mM concentration of all the three inhibitor solutions in the well and 2 µl of HK positive control was added to each well. The volume was made up to 50 µl with HK assay buffer. To each well 50 µl of the reaction mix was added as mentioned on the sigma protocol. For control samples, same procedure was followed without addition of an inhibitor solution. The samples were mixed in well by pipetting. The plate was incubated in dark for 5 min at room temperature. After 5 min (Tinitial) incubation, initial absorbance [(A450)initial] was measured at 450 nm using 96-well plate reader (Thermo Scientific). Plate was incubated for 45 min taking measurements every 10 min. Final measurement [(A450)final] and time of final reading (Tfinal) was used to calculate enzyme activity.
One unit of HK was defined as that amount of enzyme that generates 1.0 µmole of NADH per minute at pH 8.0. The HK activity achieved without any inhibitor solution was considered as 100% while HK with inhibitor solution was estimated with reference to the control sample. Samples were tested in triplicates and presented as mean ± SD. The statistical analysis was done on Graph-pad prism (version 9) software by applying students ‘t’ test with multiple comparisons.
Favipiravir was dissolved in DMSO as 2 mg/ml stock to achieve 12.73 mM concentration, while Molnupiravir stock solution was prepared in DMSO to achieve 30.39 mM concentration and 2-Deoxy-2-glucose stock solution made as 1.6 mg/ml in water. All stock solutions were diluted with water to achieve 0.1 mM concentration in the final assay reaction.
Molecular docking studies
Molecular docking of HK II with various ligands was performed by AutoDock 4.2.6 program, using the implemented empirical free energy function and the Lamarckian Genetic Algorithm (LGA). In all the dockings, a grid map with 126 × 84 × 82 points and a grid-point spacing of 1.000 Å was applied and the grid maps were calculated using AUTOGRID, version 3.0. The hydrogen bond were depicted using Discovery studio 2020 Client and Chimera softwares and interactions analyzed using Pymol software, UCSF Chimera and Accelrys Discovery Studio Visualizer software.
The analysis of the docked protein–ligand complexes was carried out for determining the comparative binding energies along with the dissociation constant (Kd) of the docked molecular complexes. The tested ligands were 2 Deoxy-d glucose, Favipiravir, Cyanrona-20 and Molnupiravir. The 3-D structure of these ligands was introduced in Pymol software for conversion of 3-D structure from SDF to PDB format. Using Pymol software, metals were also removed from the ligands structure for an appropriate docking study and further docking studies were carried out using the prepared ligands saved in PDB format. The crystal structure of target protein hexokinase II protein (PDB: 1NZT) was retrieved from Protein Data Bank (PDB) with PDB IDs and were subjected to docking process. The ligands were docked against with high Auto Dock V4.2 software.
Results
In-vitro hexokinase inhibition study
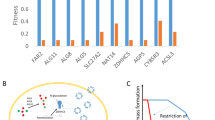
It is clear from Fig. 1, of the three drugs tested, Favipiravir is the most potent hexokinase inhibitor followed by Molnupiravir and 2DG. The status of the hexokinase inhibition by Cyan-20 and Remdesivir is not given because we did not have them at our disposal to conduct the in-vitro hexokinase inhibition experiments. Favipiravir at 0.1 mM concentration showed 50% HK II inhibition while at the same concentration, Molnupiravir and 2DG showed an inhibition of 4% and merely 0.3% respectively.
Characteristics of the drugs tested in this study
The characteristics of Favipiravir, Cyan 20, Molnupiravir, and 2DG are compared in Table 1. The EC50 value of Molnupiravir for RdRp is 0.22 µM, while it is 0.67 µM and 67.61 µM, for Remdesivir and Favipiravir respectively indicating that Molnupiravir is the most effective RdRp inhibitor (Zhao et al. 2021). The EC50 values for cyanorona-20 for SARS-COV-2 replication in Vero E6 cells, was 0.48 µM, while for Favipiravir and Remdesivir, it is reported as > 100 µM and 23.88 µM respectively (Rabie 2021). For Molnupiravir, EC50 values is against HCoV-NL63 replication with a value of 8.8 µM (Wang et al. 2021) and 0.7 mM for 2 DG (Bhatt et al. 2022). The active metabolite of Molnupiravir has a half-life of 1–1.75 h (Painter et al. 2021), and 2DG has a half-life of 1.5 h (Dwarakanath and Jain 2009). Favipiravir has a half-life of 2.5 to 5.5 h (Agrawal et al. 2020) with 54% plasma binding making it a stable molecule due to low first-pass metabolism (Wanat 2020). At a single dose of 150 mg/kg in animals (Abdelnabi et al. 2021), Molnupiravir inhibits viral multiplication by introducing errors in the covid-2 virus genome (Malone and Campbell 2021), causing mutations of 33 and 31 in viral RNA of treatment groups, respectively, while at 300 mg/kg, Favipiravir causes C-to-T and G-to-A mutations of 14 and 21, respectively (Zhirnov and Chernyshova 2021). Although, Molnupiravir is effective against SARS-CoV-2, SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), and seasonal and pandemic influenza viruses (Painter et al. 2021; Wang et al. 2021), it also targets viruses that cause equine encephalitis such as EEEV, WEEV, and VEEV (Painter et al. 2019, 2021; Wang et al. 2021) and is still being tested in humans for safety and efficacy against COVID-19 subjects (Wahl et al. 2021). Favipiravir, on the other hand, inhibits 53 types of influenza viruses, as well as Ebola, arenavirus, bunyavirus, filovirus, West Nile virus, yellow fever virus, foot-and-mouth disease virus, and Lassa virus (Furuta et al. 2013, 2017; Agrawal et al. 2020).
Molecular docking studies
The values of the net anticipated binding free energy (ΔGbind) for interactions between HK II and different ligands was calculated using several factors such as hydrogen bond (ΔGhbond), electrostatic (ΔGelec), torsional free energy (ΔGtor), and desolvation (ΔGdesolv) and are presented in Table 2. Ligand-binding affinities were predicted as negative Gibbs free energy (∆G) scores (kcal/mol), which were calculated on the basis of the AutoDock Vina scoring function. The optimal conformation with the lowest docked energy was found by the docking search. The interaction of Favipiravir with protein HK II is shown as a crystal structure in Fig. 2a while Figs. 3a, 4a and 5a and 6a shows such interactions of HKII with Molnupiravir, 2DG, Remdesivir and Cyan20, respectively. The surrounding amino acids of the HK II protein with various ligands is represented in Figs. 2b, 3b, 4b, 5b and 6b for Favipiravir, Molnupiravir, 2DG, Remdesivir and Favi-Cyan20 respectively. Panel C of Figs. 2c, 3c, 4c, 5c and 6c denotes the active site of HK II with Favipiravir, Molnupiravir, 2DG, Remdesivir and Cyan20, respectively.
Molecular docking of Favipiravir binding with Hexokinase II (PDB ID: 2NZT) shows 3D model of the interactions and the 2D interaction patterns and H-bond interactions. The polypeptide chain of HK II is folded into three structural domains, one of which is predominantly alpha-helical (red spiral) and two of which each contain a beta-pleated sheet (cyan blue) flanked by alpha-helices. The green string represents the turns of coils. Panel A shows the interaction of the ligand (Favipiravir) with protein HK II; Panel B is 3D Favipiravir with surrounding amino acids of HK II; Panel C is Favipiravir with HK II (hydrophobicity surface) at the active binding site; Panel D shows the 2D view of interaction type of Favipiravir with surrounding amino acids of HK II
Molecular docking of Crystal structure of human hexokinase II (PDB ID: 2NZT) binding with Molnupiravir shows 3D model of the interactions and the 2D interaction patterns and H-bond interaction. The polypeptide chain of HK II is folded into three structural domains, one of which is predominantly alpha-helical (red spiral) and two of which each contain a beta-pleated sheet (cyan blue) flanked by alpha-helices. The green string represents the turns of coils. Panel A shows the interaction of the ligand (Molnupiravir) with protein HK II; Panel B is Molnupiravir DG with surrounding amino acids of HK II; Panel C is Molnupiravir with HK II (hydrophobicity surface) at the active binding site; Panel D shows the 2D view of interaction type of Molnupiravir with surrounding amino acids of HK II
Molecular docking of 2-Deoxy-d-Glucose binding with Hexokinase II (PDB ID: 2NZT) shows 3D model of the interactions and the 2D interaction patterns and H-bond interaction. The polypeptide chain of HK II is folded into three structural domains, one of which is predominantly alpha-helical (red spiral) and two of which each contain a beta-pleated sheet (cyan blue) flanked by alpha-helices. The green string represents the turns of coils. Panel A shows the interaction of the ligand (2DG) with protein HK II; Panel B is 3D 2 DG with surrounding amino acids of HK II; Panel C is 2 DG with HK II (hydrophobicity surface) at the active binding site; Panel D shows the 2D view of interaction type of 2DG with surrounding amino acids of HK II
Molecular docking of Remdesivir binding with Hexokinase II (PDB ID: 2NZT) shows 3D model of the interactions and the 2D interaction patterns and H-bond interactions. The polypeptide chain of HK II is folded into three structural domains, one of which is predominantly alpha-helical (red spiral) and two of which each contain a beta-pleated sheet (cyan blue) flanked by alpha-helices. The green string represents the turns of coils. Panel A shows the interaction of the ligand (Remdesivir with protein HK II; Panel B is 3D Remdesivir with surrounding amino acids of HK II; Panel C is Remdesivir with HK II (hydrophobicity surface) at the active binding site; Panel D shows the 2D view of interaction type of Remdesivir with surrounding amino acids of HK II
Molecular docking of Cyan 20 binding with Hexokinase II (PDB ID: 2NZT) shows 3D model of the interactions and the 2D interaction patterns and H-bond interaction. The polypeptide chain of HK II is folded into three structural domains, one of which is predominantly alpha-helical (red spiral) and two of which each contain a beta-pleated sheet (cyan blue) flanked by alpha-helices. The green string represents the turns of coils. Panel A shows the interaction of the ligand (Cyan 20) with protein HK II; Panel B is 3D Cyan 20 with surrounding amino acids of HK II; Panel C is Cyan 20 with HK II (hydrophobicity surface) at the active binding site; Panel D shows the 2D view of interaction type of Cyan 20 with surrounding amino acids of HK II
It is clear from Table 2, despite having the highest binding affinity to the predicted active site of the protein (total binding energy − 5.84 kcal/mole), the favipiravir derivative—Cyan 20 formed three H-bonds with Leu734, Arg779, and Thr784 amino acid residues present at the predicted active site of the protein (Fig. 6d). Favipiravir, on the other hand, has a binding energy of − 4.68 kcal/mole, generating four hydrogen bonds with Met 119, Ile 114, Gly87, and Phe 90 (Fig. 2d). 2DG and Molnupiravir had the next highest binding energies of − 3.4 and − 3.12 kcal/mole, respectively. 2DG established two H bonds with Glu79 and Lys147 in HK II (Fig. 4d), whereas Molnupiravir showed 3 H bonds with Thr336, Ser340, and Ser415 (Fig. 3d). Remdesivir has the lowest binding energy of − 2.21 kcal/mole, making H-bonds with only two amino acids namely Lys618 and Lys510 (Fig. 5d).
Discussion
Drug developers are making efforts to consider drug repurposing as one of the more appealing choices for addressing the sudden and abrupt advent of SARS-CoV-2 that includes allopathic drugs such as Remdesivir, Dexamethasone, and Tocilizumab (Venkateshan 2021), Emodin, Omipalisib, and Tipifarnib (Jang et al. 2021; Ng et al. 2021) and selected herbal drugs (Palghadmal et al. 2021; Padmanabhan 2021; Khan and Al-Balushi 2021). Other tested medications, such as Chloroquinine, Hydroxychloroquine, and the Lopinavir-Ritonavir combination, have been withdrawn (Paul and Biswas 2021) due to inconsistent treatment reports.
COVID-19 treatment can be implemented in a variety of ways. Favipiravir, Molnupiravir, and Remdesivir are examples of drugs that affect the viral polymerase; drugs that affect COVID-19 entry to cells by inhibiting host proteases like Camostat, Nafamostat, Aprotinin, and Bromhexine; and drugs like 2DG that inhibit host hexokinase activity, which depletes the cells of energy for the virus to multiply.
When docking the 2NZT Human Hexokinase Type II with Favipiravir, it was discovered that Favipiravir displayed strongest binding energy of − 4.68 kcal/mol, followed by 2DG, Molnupiravir and Remdesivir of binding energies of − 3.4 kcal/mole, − 3.12 kcal/mol and − 2.2 kcal/mole respectively. It is interesting that the Favipiravir derivative Cyan-20 showed a binding energy of − 5.84 kcal/mole implying that Favipiarvir and its derivative formed the most stable complex with the HK enzyme since since more negative the binding energy is, the better is the ligand. The highest binding energy (most negative) was measured as the ligand with maximum binding affinity as suggested by Rafi et al. (2020). The binding energy of Favipiravir with rdrp is − 4.9 kcal/mole and its analogue6-Fluoro-4-methyl-3-oxopyrazine-2-carboxamide as − 5.1 kcal/mole (Rafi et al. 2020). With Nsp14, Favipiravir shows a binding energy of − 5.969 kcal/mole (Eweas et al. 2021) while it is − 4.8818 kcal/mole for interaction with SARS-CoV-2 3CLpro (Al-Masoudi et al. 2020). This study indicates that Favipiravir shows a binding energy value of − 4.68 kcal/mole with HKII. Docking involves the placement of a ligand within a binding site of the target protein and the prediction of the free energy of binding for such poses is calculated. Since the binding energy includes the conformational aspects of ligand and protein (Pantsar and Poso 2018), and also changes in motion (mainly an entropic effect), we believe similar binding energy of Favipiravir with different targets indicate similar binding nature.
Host esterases and anti-COVID-19 drug metabolism
Except for Favipiravir, the medicines used to treat COVID-19, like Molnupiravir, Remdesivir, and Dexamethasone, are converted to active metabolites by host esterases such as Carboxylesterase I (CES1) due to presence of ester linkages in all except, Favipiravir. It’s fascinating to learn that medications like dexamethasone stimulate CES1, one of the most abundant drug metabolizing enzymes in the human body, and therefore its conversion to active metabolite is maximized, contributing to dexamethasone’s high success rate in suppressing COVID-19 development. Furthermore, because CES1 is known to exhibit genetic variation (Merali et al. 2014), it is reasonable to predict that not every COVID-19 patient receiving Remdesivir or Molnupiravir will have the same CES1-mediated metabolism. Also, some herbal diet supplements, such as grapefruit juice, ginsenosides (Gao et al. 2020), cannabinoids, lecithin (Kang et al. 2021), and resveratrol, as well as plant extracts with active constituents such as naringenin, quercetin, luteolin, oleanolic acid, and asiatic acid, appear to have the highest CES1 inhibition potential (Qian and Markowitz 2020) which could alter the efficacy of the anti-viral drugs used.
CES1 is the most abundant hydrolase, with levels seven times higher in the human liver than in the lung, but low levels in the intestine and kidney (Li et al. 2020). However, even with Remdesivir, major side events and mortality among COVID-19 patients remained high (Wang et al. 2020). The predominant metabolite of Remdesivir (GS-5734) that reaches the lungs, GS-441524, is a potent antiviral in comparison to its parent molecule Remdesivir (Yan and Muller 2020), and the efficacy and pharmacokinetic profile of Remdesivir are compromised due to serum esterase degradation (Yan and Muller 2020; Malin et al. 2021). The cytotoxicity of Remdesivir was dramatically elevated when CES1 was overexpressed (Shen et al. 2021). It’s tempting to speculate that discrepancies in esterase expression levels in human and animals, such as rhesus monkey, dog, minipigs, rabbit, rat, and mouse plasma, are the reason for efficacy differences of the antiviral drugs in COVID-19 therapy (Bahar et al. 2012).
Albumin makes up 60% of total blood protein and has been found to have esterase activity and lower albumin levels seen in severe COVID-19 patients could potentially reduce the hydrolysis of the prodrugs to their active metabolites, which could affect the efficacy of the drugs in controlling the viral infection (Phuangsawai et al. 2014; Huang et al. 2020). Recent observations of increased albumin levels in patients with fewer negative outcomes, such as blood clot occurrences, respiratory distress, and ICU admissions, support our idea (Kheir et al. 2021). Surprisingly, viral infection reduces esterase activity in honey bees (Rinkevich et al. 2018), implying that the efficacy of medications having ester linkages, such as Molnupiravir and Remdesivir, may be affected due to lower rate of conversion of their pro drug form to active metabolite which does not apply for drugs such as Favipiravir.
Human trials of anti-COVID-19 drugs and advantages of Favipiravir over other drugs
Favipiravir has shown promising results in clinical studies in China, Russia, and Japan, with more trials in the United States, the United Kingdom, and India underway. Furthermore, two comparative trials of Favipiravir have shown that it is superior to other antivirals (Cai et al. 2020; Chen et al. 2020; Doi et al. 2020; Sanders et al. 2020; Ivashchenko et al. 2020), and other Favipiravir COVID-19 trials are currently ongoing or unreported (Doi et al. 2020; Agrawal et al. 2020). Favipiravir, appears a right drug of choice for tacking COVID-19 since it displays resistance to esterase action, has higher half-life, its safety and efficacy established, as well as the newly discovered hexokinase inhibition.
Recent reports by Wang et al. (2020) show that Remdesivir had no statistically significant clinical benefits for severe COVID-19 patients and Jang et al. (2021) demonstrate effective drug combinations like Omipalisib/Remdesivir, Tipifarnib/Omipalisib, and Tipifarnib/Remdesivir for inhibiting SARS-CoV-2 in human lung cells. Molnupiravir (MK-4482, EIDD-2801), an orally active RdRp inhibitor, is now being studied in a phase 3 clinical trial by Emory University, Ridgeback Biotherapeutics, and Merck to treat COVID-19 (Imran et al. 2021).
Hexokinase inhibitors and viruses
2-DG, on the other hand, is a glucose antimetabolite and anticancer medication that, as an analogue of glucose, enters cells and inhibits both glycolysis and glycosylation, preventing the virus from multiplying in infected cells (Halder and Mehta 2021). Scientists from the Institute of Nuclear Medicine and Allied Sciences (INMAS), India, the Defense Research and Development Organization (DRDO), India, and Dr Reddy’s Laboratories (DRL), India, have proposed an anti-COVID-19 drug after in-vitro studies revealed that this molecule effectively inhibits the growth of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus (Mantha et al. 2021).
2DG inhibits the multiplication of a number of enveloped RNA and DNA viruses through an effect on the incorporation of sugars into viral glycoproteins. At 35 mM, it inhibits the replication of Herpes Simplex Virus, while has little effect upon the replication of Newcastle disease virus or vesicular stomatitis (Scholtissek et al. 1974). Reading et al. (1998) showed that influenza load in the lung was proportional to the glucose concentration in the blood. Also, SARS-CoV-2 replication in monocytes was shown to rely entirely on ATP produced by glycolysis and multiplication of viruses such as dengue is known to require optimal glycolysis for replication (Fontaine et al. 2015). In the light of these reports, inhibition of hexokinase II appears a potential way of suppressing viral infection.
Conclusion
Our observations of in-vitro suppression of human hexokinase activity with different allopathic antiviral medicines and a few herbal antiviral agents led to the discovery of Favipiravir as a substantial inhibitor of hexokinase. Under the conditions tested, hexokinase inhibition by Favipiravir and molnupiravir showed 25 and 13 times higher levels of hexokinase inhibition than the well-known hexokinase inhibitor 2 Deoxy d glucose (2DG). The in-vitro data is supported by the molecular docking studies that reflect that Favipiravir is the most potent ligand for a robust contact with Hexokinase II followed by Molnupiravir and 2 DG. This data shows that Favipiravir has the lowest amount of viral replication suppression, but because of its hexokinase inhibitory property, one could assume an additional mode of action of hexokinase inhibition, making this molecule more powerful than the other molecules examined.
References
Abdelnabi R, Foo CS, Kaptein SJF et al (2021) The combined treatment of Molnupiravir and Favipiravir results in a marked potentiation of efficacy 1 in a SARS-CoV2 hamster infection model through an increased frequency of mutations in the viral 2 genome. EBioMedicine 72:103595
Abuga K, Nyamweya N (2021) Alcohol-based hand sanitizers in COVID-19 prevention: a multidimensional perspective. Pharmacy 9:64
Agrawal U, Raju R, Udwadia ZF (2020) Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India 76:370–376
Ajaz S, McPhail MJ, Singh KK et al (2021) Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol 320:C57–C65
Al-Masoudi NA, Elias RS, Saeed B (2020) Molecular docking studies of some antiviral and antimalarial drugs via bindings to 3CL-protease and polymerase enzymes of the novel coronavirus (SARSCoV-2). Biointerface Res Appl Chem 10:6444–6459
Al-Ziaydi AG, Al-Shammari AM, Hamzah MI et al (2020) Hexokinase inhibition using D-Mannoheptulose enhances oncolytic newcastle disease virus-mediated killing of breast cancer cells. Cancer Cell Int 20:420
Bahar FG, Ohura K, Ogihara T et al (2012) Species difference of esterase expression and hydrolase activity in plasma. J Pharm Sci 101:3979–3988
Bhatt AN, Kumar A, Rai Y et al (2022) Glycolytic inhibitor 2-Deoxy-D-glucose attenuates SARS-CoV-2 multiplication in host cells and weakens the infective potential of progeny virions. Life Sci 295:120411
Cai Q, Yang M, Liu D et al (2020) Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering 6:1192–1198
Chen C, Zhang Y, Huang J, Yin P et al (2020) Favipiravir versus arbidol for COVID-19: a randomized clinical trial. Front Pharmacol 12:683296
Chien M, Anderson TK, Jockusch S et al (2020) Nucleotide analogues as Inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res 19(11):4690–4697
Courteau L, Crasto J, Hassanzadeh G et al (2015) Hexokinase 2 controls cellular stress response through localization of an RNA-binding protein. Cell Death Dis 6:e1837
Darazam IA, Shokouhi S, Pourhoseingholi MA et al (2021) Role of interferon therapy in severe COVID-19: the COVIFERON randomized controlled trial. Sci Rep 11:8059
Doi K, Ikeda M, Hayase N et al (2020) Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID-19: a case series. Crit Care 24(1):392
Dwarakanath BS, Jain V (2009) Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol 5:581–585
Eweas AF, Alhossary AA, Abdel-Moneim AS (2021) Molecular docking reveals Ivermectin and Remdesivir as potential repurposed drugs against SARS-CoV-2. Front Microbiol 11:592908
Fontaine KA, Sanchez EL, Camarda R et al (2015) Dengue virus induces and requires glycolysis for optimal replication. J Virol 89:2358–2366
Forrestall KL, Burley DE, Cash MK et al (2021) 2-Pyridone natural products as inhibitors of SARS-CoV-2 main protease. Chem Biol Interact 335:109348
Furuta Y, Gowen BB, Takahashi K et al (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivirus Res 100:446–454
Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B 93:449–463
Gao H, Liang D, Li C et al (2020) 2-Deoxy-Rh2: a novel ginsenoside derivative, as dual-targeting anti-cancer agent via regulating apoptosis and glycolysis. Biomed Pharmacother 124:109891
Girum T, Lentiro K, Geremew M et al (2020) Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Trop Med Health 48:91
Halder S, Mehta AK (2021) 2-Deoxy-D-glucose: is this the final cure for COVID-19: or yet another mirage? Eur Rev Med Pharmacol Sci 25:4448–4450
Huang J, Cheng A, Kumar R et al (2020) Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol 92:2152–2158
Icard P, Lincet H, Wu Z et al (2021) The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response. Biochimie 180:169–177
Imran M, Arora MK, Asdaq SMB et al (2021) Discovery, development, and patent trends on Molnupiravir: a prospective oral treatment for COVID-19. Molecules 26:5795
Ivashchenko AA, Dmitriev KA, Vostokova NV et al (2020) AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis 73:531–534
Jang WD, Jeon S, Kim S et al (2021) Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay. PNAS 118:e2024302118
Kang J-H, Kim Y-J, Yang M-S et al (2021) Co-Spray dried Nafamostat mesylate with lecithin and mannitol as respirable microparticles for targeted pulmonary delivery: pharmacokinetics and lung distribution in rats. Pharmaceutics 13:1519
Khan SA, Al-Balushi K (2021) Combating COVID-19: the role of drug repurposing and medicinal plants. J Infect Public Health 14:495–503
Kheir M, Saleem F, Wang C et al (2021) Higher albumin levels on admission predict better prognosis in patients with confirmed COVID-19. PLoS ONE 16:e0248358
Li J-Y, You Z, Wang Q et al (2020) The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect 22:80–85
Malin JJ, Suárez I, Priesner V et al (2021) Remdesivir against COVID-19 and other viral diseases. Clin Microbiol Rev 34:e00162-e220
Malone B, Campbell EA (2021) Molnupiravir: coding for catastrophe. Nat Struct Mol Biol 28:706–711
Mantha MK, Suvvari TK, Corriero AC (2021) 2-Deoxy-D-glucose as an armament against COVID-19: the key to return to normality? Biomed Biotechnol Res J 5:347–348
Mengist HM, Dilnessa T, Jin T (2021) Structural basis of potential inhibitors targeting SARS-CoV-2 main protease. Front Chem 9:622898
Merali Z, Ross S, Paré G (2014) The pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effect. Drug Metab Drug Interact 29:143–151
Ng YL, Salim CK, Chu JJH (2021) Drug repurposing for COVID-19: approaches, challenges and promising candidates. Pharmacol Ther 228:107930
Nicola M, Alsafi Z, Sohrabi C et al (2020) The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 78:185–193
Padmanabhan S (2021) Potassium levels in COVID subjects: current observations and new possibilities for its use in COVID diagnosis. Glob J Med Res 21:1–8
Painter GR, Bowen RA, Bluemling GR et al (2019) The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal venezuelan equine encephalitis virus Infection. Antiviral Res 171:104597
Painter WP, HolmanW BJA et al (2021) Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother 65:e02428-e2520
Palghadmal SB, Kulkarni PS, Makadia V et al (2021) Tackling complications of coronavirus infection with quercetin: observations and hypotheses. Explor Res Hypothesis Med 6:193–204
Pantsar T, Poso A (2018) Binding affinity via docking: fact and fiction. Molecules 23:1899
Paul SS, Biswas G (2021) Repurposed antiviral drugs for the treatment of COVID-19: syntheses, mechanism of infection and clinical trials. Mini Rev Med Chem 21:1123–1143
Phuangsawai O, Hannongbua S, Gleeson MP (2014) Elucidating the origin of the esterase activity of human serum albumin using QM/MM calculations. J Phys Chem B 118:11886–11894
Prajapati P, Desai H, Chandarana C (2022) Hand sanitizers as a preventive measure in COVID-19 pandemic, its characteristics, and harmful effects: a review. J Egypt Public Health Assoc 97:6
Qian Y, Markowitz JS (2020) Natural products as modulators of CES1 activity. Drug Metab Dispos 48:993–1007
Rabie AM (2021) Discovery of (E)-N-(4-cyanobenzylidene)-6-fluoro-3-hydroxypyrazine-2-carboxamide (cyanorona-20): the first potent and specific anti-COVID-19 drug. Chem Pap 75:4669–4685
Rafi MdO, Bhattacharje G, Al-Khafaji K et al (2020) Combination of QSAR, molecular docking, molecular dynamic simulation and MM-PBSA: analogues of lopinavir and favipiravir as potential drug candidates against COVID-19. J Biomol Struct Dyn 2020:1–20
Ramière C, Rodriguez J, Enache LS et al (2014) Activity of hexokinase is increased by its interaction with hepatitis C virus protein NS5A. J Virol 88:3246–3254
Reading PC, Allison J, Crouch EC et al (1998) Increased susceptibility of diabetic mice to Influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol 72:6884–6887
Rinkevich FD, Margotta JW, Simone-Finstrom M et al (2018) Esterase activity is affected by genetics, age, insecticide exposure, and viral infection in the honey bee, Apis mellifera. Biorxiv
Sagawa T, Inoue KI, Takano H (2020) Use of protease inhibitors for the prevention of COVID-19. Prev Med 141:106280
Sahu KK, Kumar R (2021) Role of 2-Deoxy-D-Glucose (2-DG) in COVID-19 disease: a potential game-changer. J Fam Med Prim Care 10(10):3548–3552
Saha S, Nandi R, Vishwakarma P et al (2021) Discovering potential RNA dependent RNA polymerase inhibitors as prospective drugs against COVID-19: an in silico approach. Front Pharmacol 12:634047
Sanders JM, Monogue ML, Jodlowski TZ et al (2020) Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19). A review. JAMA 323:1824–1836
Scholtissek C, Rott R, Hau G et al (1974) Inhibition of the multiplication of vesicular stomatitis and Newcastle disease virus by 2-deoxy-D-glucose. J Virol 13:1186–1193
Seth S, Batra J, Srinivasan S (2020) COVID-19: Targeting proteases in viral invasion and host immune response. Front Mol Biosci 7:215
Shang Y, Li H, Zhang R (2021a) Effects of pandemic outbreak on economies: evidence from business history context. Front Public Health 9:632043
Shang C, Zhuang X, Zhang H et al (2021b) Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice. Virology J 18:46
Shen T, Wang T (2021) Metabolic reprogramming in COVID-19. Int J Mol Sci 22:11475
Shen Y, Eades W, Yan B (2021) The COVID-19 medicine Remdesivir is therapeutically activated by carboxylesterase-1, and excessive hydrolysis increases cytotoxicity. Hepatol Commun 5:1622–1623
Su H-X, Yao S, Zhao, W-F et al (2020) Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin 1–11
Takashita E, Kinoshita N, Yamayoshi S et al (2022) Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant. N Engl J Med 386:10
Tempestilli M, Caputi P, Avataneo V et al (2020) Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother 75:2977–2980
Teralı K, Baddal B, Gülcan HO (2020) Prioritizing potential ACE2 inhibitors in the COVID-19 pandemic: Insights from a molecular mechanics-assisted structure-based virtual screening experiment. J Mol Graph Model 100:107697
Vangeel L, Chiu W, De Jonghe S et al (2022) Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivirus Res 198:105252
Venkatesan P (2021) Repurposing drugs for treatment of COVID-19. Lancet Respir Med 9:E63
Wahl A, Gralinski LE, Johnson CE et al (2021) SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 591:451–457
Wanat K (2020) Biological barriers, and the influence of protein binding on the passage of drugs across them. Mol Biol Rep 47:3221–3231
Wang Y, Zhang D, Du G et al (2020) Remdesivir in adults with severe COVID-19: a randomized, double blind, placebo-controlled, multicenter trial. Lancet 395:1569–1578
Wang Y, Li P, Solanki K et al (2021) Viral polymerase binding and broad-spectrum antiviral activity of molnupiravir against human seasonal coronaviruses. Virology 564:33–38
Yan VC, Muller FL (2020) Advantages of the parent nucleoside GS-441524 over Remdesivir for COVID-19 treatment. ACS Med Chem Lett 11:1361–1366
Zhao J, Guo SS, Yi D et al (2021) A cell-based assay to discover inhibitors of SARS-CoV-2 RNA dependent RNA polymerase. Antiviral Res 190:105078
Zhirnov OP, Chernyshova AI (2021) Favipiravir: the hidden threat of mutagenic action. J Microbiol Epidemiol Immunobiol 98:213–220
Acknowledgements
The authors thank Mr. Vinod Jadhav, Chairman Sava Healthcare Limited (SHL), and Mr. Dinesh Kapoor, CEO, SHL for their constant support and encouragement. Thanks are also due to Mr. Bhaskar Musmade, Manager, Analytical Development Laboratory, SAVA R & D, for HPLC analysis of the drugs used in the in-vitro hexokinase inhibition study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PK carried out hexokinase inhibition studies and statistical analysis of the generated data while SP was involved in conception, design, data analysis and drafting of the manuscript. Both the authors approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interests.
Research involving animal rights
This article does not contain any studies with animals performed by either of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kulkarni, P., Padmanabhan, S. A novel property of hexokinase inhibition by Favipiravir and proposed advantages over Molnupiravir and 2 Deoxy d glucose in treating COVID-19. Biotechnol Lett 44, 831–843 (2022). https://doi.org/10.1007/s10529-022-03259-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03259-6