Abstract
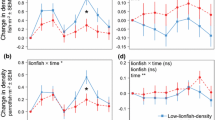
Range expanding species can have major impacts on marine ecosystems but experimental field based studies are often lacking. The urchin Centrostephanus rodgersii has recently undergone a southerly range expansion to the east coast of Tasmania, Australia. We manipulated densities of C. rodgersii and algal regrowth in urchin barrens habitat to test effects of the urchin on biotic interactions between two native herbivores, black-lip abalone (Haliotis rubra) and another urchin (Heliocidaris erythrogramma), and their benthic habitat. After 13 months, removals of only C. rodgersii resulted in overgrowth of barrens habitat by algae and sessile invertebrates. Densities of abalone increased (+92 %) only in patches from which C. rodgersii was removed and algal regrowth allowed. In contrast, densities of H. erythrogramma increased in all treatments (+45, +28, +25 %) in which C. rodgersii was removed, irrespective of the algal regrowth manipulations. These results suggest that C. rodgersii has a negative influence on the densities of abalone through competition for food and on densities of H. erythrogramma through competition for preferred habitat. Densities of abalone (+65 %) but not H. erythrogramma (+25 %), were lower in the patches from which C. rodgersii and canopy algae regrowth were removed relative to patches from which only C. rodgersii was removed (+92 and +28 %, respectively). These results suggest that C. rodgersii overgrazing of canopy-algae results in loss of structural complexity which could increase abalone susceptibility to predation, cause abalone to seek shelter in cryptic microhabitats and/or prevent their return to patches where canopy algae are absent. The ongoing spread of C. rodgersii and expansion of barrens habitat in eastern Tasmania will continue to negatively affect populations of these two native herbivores and their associated fisheries at a range of spatial scales. This example shows that habitat modifying species which become highly invasive can have disproportionate negative impacts on the structure and dynamics of the recipient community.





Similar content being viewed by others
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMOVA for primer: guide to software and statistical methods, 1st edn. PRIMER-E, Plymouth
Andrew NL (1993) Spatial heterogeneity, urchin grazing, and habitat structure on reefs in temperate Australia. Ecology 74:292–302
Andrew NL, Underwood AJ (1992) Associations and density of urchins and abalone on shallow subtidal reefs in southern New South Wales. Mar Freshw Res 43:547–1559
Andrew NL, Worthington DG, Brett PA, Bentley N R, Chick C, Blount C (1998) Interactions between the abalone fishery and urchins in New South Wales. Final Report Series No.12, NSW fisheries, Sydney
Bertness MD (1984) Habitat and community: modification by an introduced herbivorous snail. Ecology 65:370–381
Cai W (2006) Antarctic ozone depletion causes an intensification of the Southern Ocean super-gyre circulation. Geophys Res Lett 33:L03712. doi:10.1029/2005GL024911
Cai W, Shi G, Cowan T, Bi D, Ribbe J (2005) The response of the Southern Annular Mode, the East Australian Current, and the southern mid-latitude ocean circulation to global warming. Geophy Res Lett 32: L23706, 1–4
Chapman ARO (1981) Stability of urchin dominated barren grounds following destructive grazing of kelp in St. Margaret’s Bay, Eastern Canada. Mar Biol 62:307–311
Chapman ARO, Johnson CR (1990) Disturbance and organization of macroalgal assemblages in the northwest Atlantic. Hydrobiologia 192:77–122
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Connolly RM (1986) Behaviour and ecology of the urchin Heliocidaris erythrogramma (Valenciennes). B.Sc. Hons thesis. University of Adelaide, Adelaide
Day E, Branch GM (2000) Evidence for a positive relationship between juvenile abalone Haliotis midae and the urchin Parechinus angulosus in the south western Cape, South Africa. S Afri J Mar Sci 22:145–156
Duggins DO, Simenstad CA, Estes JA (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245:170–173
Edgar GJ (1997) Australian marine life: the plants and animals of temperate waters. Reed New Holland, Sydney
Edgar GJ, Barrett SN, Morton A, Samson RC (2004) Effects of algal canopy clearance on plant, fish and macroinvertebrate communities on eastern Tasmanian reefs. J Exp Mar Biol Ecol 312:67–87
Firth LB, Crowe TP, Moore P, Thompson RC, Hawkins SJ (2009) Predicting impacts of climate-induced range expansion: an experimental framework and a test involving key grazers on temperate rocky shores. Glob Chang Biol 15:1413–1421
Fletcher WJ (1987) Interactions among subtidal Australian urchins, gastropods and algae: effects of experimental removal. Ecol Monogr 57:89–109
Flukes EB, Johnson CR, Ling SD (2012) Forming sea urchin barrens from the inside out: an alternative pattern of overgrazing. Mar Ecol Prog Ser 464:179–194
Graham MH (2004) Effects of local deforestation of the diversity and structure of southern California giant kelp forests food webs. Ecosystems 7:341–357
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol 17(1):22–27
Harley CDG, Hughs RA, Hulgren KM, Miner BF, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impact of climate change in coast marine systems. Ecol Lett 9:228–241
Harrold C, Reed DC (1985) Food availability, urchin grazing, and kelp forest community structure. Ecology 66:1160–1169
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Ann Rev Ecol Evol Syst 37:373–404
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Chang Biol 12:450–455
Hollebone AL, Hay ME (2008) An invasive crab alters interaction webs in a marine community. Biol Invasions 10:347–358
Johnson C, Ling S, Ross J, Scoresby S, Miller K (2005) Establishment of the long-spined urchin (Centrostephanus rodgersii) in Tasmania: first assessment of the potential threats to fisheries. Final report, Fisheries Research Development Corporation, Hobart, Tasmania
Johnson CR, Banks S, Barrett NS, Cazassus F, Dunstan PK, Edgar GJ, Frusher SD, Gardner C, Haddon M et al (2011) Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J Exp Mar Biol Ecol 400(1–2):17–32
Keesing J (2006) Ecology of Heliocidaris erythrogramma. In: Lawrence JM (ed) Edible urchins: biology and ecology, 2nd edn. Elsevier, New York, pp 329–341
Konar B (2000) Seasonal inhibitory effects of marine plants on urchins: structuring communities the algal way. Oecologia 125(2):208–217
Lawrence JM (1975) On the relationship between marine plants and urchins. Ocean Mar Biol Ann Rev 13:213–286
Ling SD (2008) Range expansion of a habitat modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156(4):883–894
Ling SD, Johnson CR, Ridgway K, Hobday AJ, Haddon M (2009) Climate driven range extension of a urchin: inferring future trend by analysis of recent population dynamics. Glob Chang Biol 15:719–731
Ling SD, Ibbott S, Sanderson JC (2010) Recovery of canopy-forming macroalgae following removal of the enigmatic grazing urchin Heliocidaris erythrogramma. J Exp Mar Biol Ecol 395:135–146
McClanahan TR (1988) Coexistence in a urchin guild and its implications to coral reef diversity and degradation. Oecologia 77:210–218
Naylor R, Gerring P (2001) Interaction between pauna and kina. Water Atmos 9:16–17
O’Connor NE, Crowe TP (2005) Biodiversity loss and ecosystem functioning: distinguishing between number and identity of species. Ecology 86:1783–1796
Parmesan C, Yohe G (2003) A globally coherent fingerprint of change. Nature 421:37–42
Pederson HG, Johnson CR (2006) Predation of the urchin Heliocidaris erythrogramma by rock lobsters (Jasus edwardsii) in no-take marine reserves. J Exp Mar Biol Ecol 336:120–134
Pederson HG, Barrett ND, Frusher SD, Buxton CD (2008) Effect of predator-prey and competitive interactions on size at emergence in the black-lip abalone Haliotis rubra in a Tasmania MPA. Mar Ecol Prog Ser 366:91–98
Pinnegar JK, Polunin NVC, Francour P, Badalamenti F, Chemello R, Harmelin-Vivien ML, Hereu B, Milazzo M et al (2000) Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ Conserv 27:179–200
Poloczanska ES, Hawkins SJ, Southward AJ, Burrows MT (2008) Modelling the response of populations of competing species to climate change. Ecology 89:3138–3149
Ridgway K (2007) Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophys Res Lett 34:L13613
R Development Core Team (2010) R: a language and environment for statistical computing. R project for statistical computing, Vienna, Austria. http://www.R-project.org
Sauchyn LK, Scheibling RE (2009) Degradation of urchin feces in a rocky subtidal ecosystem: implications for nutrient cycling and energy flow. Aquat Biol 6:99–108
Shepherd SA (1973) Competition between urchins and abalone. Aust Fish 4:4–7
Shulman MJ (1990) Aggression among urchins on Caribbean coral reefs. J Exp Mar Biol Ecol 140:197–207
Sorte CJB, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts. Glob Ecol Biol 19:303–316
Strain EMA, Johnson CR (2009) Competition between an invasive urchin and commercially fished abalone: effect on body condition, reproduction and survivorship. Mar Ecol Prog Ser 377:169–182
Stuart-Smith RD, Barrett NS, Stevenson DG, Edgar GJ (2009) Stability in temperate reef communities over a decadal time scale despite concurrent ocean warming. Glob Chang Biol 16(1):122–134
Tegner MJ, Dayton PK (2000) Ecosystem effects of fishing in kelp forest communities. ICES J Mar Res 57:579–589
Tegner MJ, Levin LL (1982) Do urchins and abalone compete in the California kelp communities? In: Lawrence J (ed) International echinoderms conference. A.A. Balkema, Rotterdam, pp 265–271
Todd CD, Keough MJ (1994) Larval settlement in hard substratum epifaunal assemblages: a manipulative field study of the effects of substratum filming and the presence of incumbents. J Exp Mar Biol Ecol 181:159–187
Valentine JP, Johnson C (2003) Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol 295:63–90
Vanderklift MA, Kendrick GA (2005) Contrasting influence of urchins on attached and drift macroalgae. Mar Ecol Prog Ser 299:101–110
Vanderklift MA, Wernberg T (2008) Detached kelps from distant sources are a food subsidy for urchins. Oecologia 157:327–335
Walther G (2010) Community and ecosystem responses to recent climate change. Philos Trans R Soc B 365:2019–2024
Williams AH (1977) Three-way competition in a patchy back reef environment. PhD thesis University of North Carolina
Williams SL, Grosholz ED (2008) The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuaries Coasts 31:3–20
Acknowledgments
We thank those who assisted with fieldwork, particularly Ryan Downie, Richard Holmes and David Sinn. We thank Dr. Graham Edgar and Dr. Adriana Villamor for their useful comments on an earlier draft of the manuscript. This study was part of a Commonwealth Scientific and Industrial Research Organization Joint PhD Program in Quantitative Marine Science and supported by an Australian Postgraduate Award. The research was supported by Tasmanian Aquaculture and Fisheries Institute, and Tasmanian Abalone Council grants.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Strain, E.M.A., Johnson, C.R. The effects of an invasive habitat modifier on the biotic interactions between two native herbivorous species and benthic habitat in a subtidal rocky reef ecosystem. Biol Invasions 15, 1391–1405 (2013). https://doi.org/10.1007/s10530-012-0378-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0378-7