Abstract
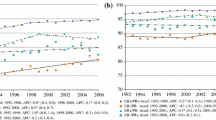
The current therapeutic strategy in breast cancer is to identify a target, such as estrogen receptor (ER) status, for tailoring treatments. We investigated the patterns of recurrence with respect to ER status for patients treated in two randomized trials with 25 years’ median follow-up. In the ER-negative subpopulations most breast cancer events occurred within the first 5–7 years after randomization, while in the ER-positive subpopulations breast cancer events were spread through 10 years. In the ER-positive subpopulation, 1 year endocrine treatment alone significantly prolonged disease-free survival (DFS) with no additional benefit observed by adding 1 year of chemotherapy. In the small ER-negative subpopulation chemo-endocrine therapy had a significantly better DFS than endocrine alone or no treatment. Despite small numbers of patients, “old-fashioned” treatments, and competing causes of treatment failure, the value of ER status as a target for response to adjuvant treatment is evident through prolonged follow-up.

Similar content being viewed by others
References
Goldhirsch A, Wood W, Gelber R et al (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer. Ann Oncol 18:1133–1144. doi:10.1093/annonc/mdm271
Zava DT, Wyler-Von Ballmoos A, Goldhirsch A et al (1982) A quality control study to assess the inter-laboratory variability of routine estrogen and progesterone receptor assays. Eur J Cancer Clin Oncol 18:713–721. doi:10.1016/0277-5379(82)90068-2
Jordan VC, Zava DT, Eppenberger U et al (1983) Reliability of steroid hormone receptor assays: an international study. Eur J Cancer Clin Oncol 19:357–363. doi:10.1016/0277-5379(83)90133-5
Goldhirsch A, Coates AS, Gelber RD et al (2006) First select the target: better choice of adjuvant treatments for breast cancer patients. Ann Oncol 17:1772–1776. doi:10.1093/annonc/mdl398
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717. doi:10.1016/S0140-6736(05)66544-0
Ludwig Breast Cancer Study Group (1984) Randomized trial of chemoendocrine therapy, endocrine therapy, and mastectomy alone in postmenopausal patients with operable breast cancer and axillary node metastasis. Lancet 1:1256–1260
Goldhirsch A, Gelber RD, Simes RJ et al (1989) Costs and benefits of adjuvant therapy in breast cancer: a quality-adjusted survival analysis. J Clin Oncol 7:36–44
Castiglione M, Gelber RD, Goldhirsch A (1990) Adjuvant systemic therapy for breast cancer in the elderly: competing causes of mortality. J Clin Oncol 8:519–526
Castiglione-Gertsch M, Johnsen C, Goldhirsch A et al (1994) The International (Ludwig) Breast Cancer Study Group Trials I–IV: 15 years follow-up. Ann Oncol 5:717–724
Crivellari D, Price K, Gelber RD et al (2003) Adjuvant endocrine therapy compared with no systemic therapy for elderly women with early breast cancer: 21-year results of International Breast Cancer Study Group Trial IV. J Clin Oncol 21:4517–4523. doi:10.1200/JCO.2003.03.559
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481. doi:10.2307/2281868
Gray RJ (1988) A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154. doi:10.1214/aos/1176350951
Kalbfleisch JD, Prentice RL (1980) The statistical analysis of failure time data. Wiley, New York, p 169
Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14:2738–2746
Goss P, Allan AL, Rodenhiser DI et al (2008) New clinical and experimental approaches for studying tumour dormancy: does tumour dormancy offer a therapeutic target? APMIS 116:552–568
Clough-Gorr KM, Fink AK, Silliman RA (2008) Challenges associated with longitudinal survivorship research: attrition and a novel approach of reenrollment in a 6-year follow-up study of older breast cancer survivors. J Cancer Surviv 2(2):95–103. doi:10.1007/s11764-008-0049-y
Cufer T (2007) Reducing the risk for breast cancer recurrence after completion of tamoxifen treatment in postmenopausal women. Ann Oncol Suppl 8:viii8–viii17
Swaby RF, Jordan VC (2008) Low-dose estrogen therapy to reverse acquired antihormonal resistance in the treatment of breast cancer. Clin Breast Cancer 8(2):124–133. doi:10.3816/CBC.2008.n.012
Berry DA, Cirrincione C, Henderson IC et al (2006) Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658–1667. doi:10.1001/jama.295.14.1658
Albain K, Barlow W, O’Malley F et al (2004) Mature outcomes and new biologic correlates on phase III Intergroup trial 0100 (INT-0100, SWOG-8814): concurrent (CAFT) vs sequential (CAF-T) chemohormonal therapy (cyclophosphamide, doxorubicin, 5-fluorouracil, tamoxifen) vs T alone for postmenopausal, node positive, estrogen (ER) and/or progesterone PgR receptor-positive breast cancer. Proc San Antonio Breast Cancer Symposium, 2004 (Abstr 37)
Hamilton A, Hortobagyi G (2005) Chemotherapy: what progress in the last 5 years? J Clin Oncol 23:1760–1775. doi:10.1200/JCO.2005.10.034
Breast International Group MINDACT (Microarray In Node negative Disease may Avoid Chemotherapy) trial. http://www.breastinternationalgroup.org/TransBIG/Mindact.aspx. Accessed October 3, 2008
National Cancer Institute US National Institutes of Health. The TAILORx Breast Cancer Trial. http://www.cancer.gov/clinicaltrials/digestpage/TAILORx. Accessed October 3, 2008
Acknowledgments
We thank the patients, physicians, nurses, and data managers who participate in the International Breast Cancer Study Group trials. We thank Rita Hinkle for data management and IBCSG patients and participants who have submitted yearly trial patient data for 30 years: West Swedish Breast Cancer Group; Institute of Oncology, Ljubljana, Slovenia; Swiss Group for Clinical Cancer Research (SAKK); Australian New Zealand Breast Cancer Trials Group (ANZ BCTG); Groote Shuur Hospital, Cape Town, South Africa. Funding (in addition to above) provided by: Ludwig Institute for Cancer Research, Swedish Cancer Society, The Cancer Council Australia, National Health Medical Research Council of Australia, Frontier Science and Technology Research Foundation, US-National Cancer Institute (CA-75362), Cancer Association of South Africa (CANSA), and Foundation of Clinical Cancer Research of Eastern Switzerland (OSKK).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Pagani, O., Price, K.N., Gelber, R.D. et al. Patterns of recurrence of early breast cancer according to estrogen receptor status: a therapeutic target for a quarter of a century. Breast Cancer Res Treat 117, 319–324 (2009). https://doi.org/10.1007/s10549-008-0282-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0282-0