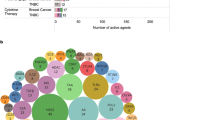
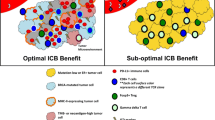
Abstract
Immune checkpoint inhibitors have modified the treatment algorithm in a variety of cancer types, including breast cancer. Nevertheless, optimal selection of ideal candidates to these drugs remains an unmet need. Although PD-L1 expression by immunohistochemistry seems to be the most promising biomarker to date, its predictive ability is far from ideal. Thus, the development of new predictive biomarkers is essential for a better selection of patients. Here, we discuss potential biomarkers beyond PD-L1 that could play an important role in precision cancer immunotherapy.

Similar content being viewed by others
References
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348:56–61. https://doi.org/10.1126/SCIENCE.AAA8172
Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810–821. https://doi.org/10.1056/nejmoa1910549
Loibl S, Untch M, Burchardi N et al (2019) A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 30:1279–1288. https://doi.org/10.1093/annonc/mdz158
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Schmid P, Adams S, Rugo HS et al (2019) IMpassion130: updated overall survival (OS) from a global, randomized, double-blind, placebo-controlled, Phase III study of atezolizumab (atezo) + nab- paclitaxel (nP) in previously untreated locally advanced or metastatic triple-negative breast ca. J Clin Oncol 37:1003–1003. https://doi.org/10.1200/JCO.2019.37.15_suppl.1003
Cortes J, Cescon DW, Rugo HS et al (2020) KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol 38:1000–1000. https://doi.org/10.1200/jco.2020.38.15_suppl.1000
Cortes J, Cescon DW, Rugo HS et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396:1817–1828. https://doi.org/10.1016/S0140-6736(20)32531-9
Rugo HS, Loi S, Adams S et al (2019) Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): post-hoc analysis of IMpassion130. Ann Oncol 30:v858–v859. https://doi.org/10.1093/annonc/mdz394.009
Schmid P, Salgado R, Park YH et al (2020) Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. https://doi.org/10.1016/j.annonc.2020.01.072
Sunshine J, Taube JM (2015) PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 23:32. https://doi.org/10.1016/J.COPH.2015.05.011
Brasó-Maristany F, Sansó M, Chic N et al (2021) Case report: a case study documenting the activity of atezolizumab in a PD-L1-negative triple-negative breast cancer. Front Oncol. https://doi.org/10.3389/FONC.2021.710596
Mittendorf EA, Zhang H, Barrios CH et al (2020) Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396:1090–1100. https://doi.org/10.1016/S0140-6736(20)31953-X
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271. https://doi.org/10.1093/ANNONC/MDU450
Denkert C, von Minckwitz G, Darb-Esfahani S et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Stanton SE, Adams S, Disis ML (2016) Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2:1354–1360. https://doi.org/10.1001/JAMAONCOL.2016.1061
Loi S, Adams S, Schmid P et al (2017) Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol 28:v608. https://doi.org/10.1093/ANNONC/MDX440.005
Winer EP, Lipatov O, Im SA et al (2021) Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 22:499–511. https://doi.org/10.1016/S1470-2045(20)30754-3
Emens LA, Molinero L, Loi S et al (2021) Atezolizumab and nab -paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. JNCI J Natl Cancer Inst 113:1005–1016. https://doi.org/10.1093/jnci/djab004
Loi S, Giobbie-Hurder A, Gombos A et al (2019) Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol 20:371–382. https://doi.org/10.1016/S1470-2045(18)30812-X
Emens LA, Esteva FJ, Beresford M et al (2020) Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol 21:1283–1295. https://doi.org/10.1016/S1470-2045(20)30465-4
Salgado R, Denkert C, Campbell C et al (2015) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 1:448–455. https://doi.org/10.1001/jamaoncol.2015.0830
Denkert C, von Minckwitz G, Brase JC et al (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2–positive and triple-negative primary breast cancers. J Clin Oncol 33:983–991. https://doi.org/10.1200/JCO.2014.58.1967
Heppner BI, Untch M, Denkert C et al (2016) Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res 22:5747–5754. https://doi.org/10.1158/1078-0432.CCR-15-2338
Issa-Nummer Y, Darb-Esfahani S, Loibl S et al (2013) Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer—a substudy of the neoadjuvant geparquinto trial. PLoS ONE 8:e79775. https://doi.org/10.1371/JOURNAL.PONE.0079775
Nuciforo P, Pascual T, Cortés J et al (2018) A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol 29:170–177. https://doi.org/10.1093/annonc/mdx647
Chic N, Luen SJ, Nuciforo P et al (2021) Tumor cellularity and infiltrating lymphocytes as a survival surrogate in HER2-positive breast cancer. JNCI J Natl Cancer Inst. https://doi.org/10.1093/JNCI/DJAB057
Kim R, Song N, Gavin P et al (2019) Stromal tumor-infiltrating lymphocytes in NRG oncology/NSABP B-31 adjuvant trial for early-stage HER2-positive breast cancer. J Natl Cancer Inst 111:867–871. https://doi.org/10.1093/JNCI/DJZ032
Krop IE, Paulson J, Campbell C et al (2019) Genomic correlates of response to adjuvant trastuzumab (H) and pertuzumab (P) in HER2+ breast cancer (BC): biomarker analysis of the APHINITY trial. J Clin Oncol 37:1012–1012. https://doi.org/10.1200/JCO.2019.37.15_SUPPL.1012
Loi S, Michiels S, Salgado R et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25:1544–1550. https://doi.org/10.1093/ANNONC/MDU112
Criscitiello C, Vingiani A, Maisonneuve P et al (2020) Tumor-infiltrating lymphocytes (TILs) in ER+/HER2− breast cancer. Breast Cancer Res Treat 183:347–354. https://doi.org/10.1007/s10549-020-05771-7
Park JH, Jonas SF, Bataillon G et al (2019) Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol 30:1941–1949. https://doi.org/10.1093/ANNONC/MDZ395
De Jong VMT, Wang Y, Opdam M et al (2020) 159O Prognostic value of tumour infiltrating lymphocytes in young triple negative breast cancer patients who did not receive adjuvant systemic treatment; by the PARADIGM study group. Ann Oncol 31:S303. https://doi.org/10.1016/J.ANNONC.2020.08.281
Bianchini G, Huang C-S, Egle D et al (2020) LBA13 tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann Oncol 31:S1145–S1146. https://doi.org/10.1016/J.ANNONC.2020.08.2241
Loi S, Dushyanthen S, Beavis PA et al (2016) RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 22:1499–1509. https://doi.org/10.1158/1078-0432.CCR-15-1125
Dieci MV, Radosevic-Robin N, Fineberg S et al (2018) Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International immuno-oncology biomarker working group on breast cancer. Semin Cancer Biol 52:16–25
Adams S, Diamond JR, Hamilton E et al (2019) Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol 5:334–342. https://doi.org/10.1001/JAMAONCOL.2018.5152
Asano Y, Kashiwagi S, Goto W et al (2016) Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg. https://doi.org/10.1002/bjs.10127
De AC, Nagi C, Hoyt CC et al (2020) Evaluation of the predictive role of tumor immune infiltrate in patients with HER2-positive breast cancer treated with neoadjuvant anti-HER2 therapy without chemotherapy. Clin Cancer Res 26:738–745. https://doi.org/10.1158/1078-0432.CCR-19-1402
Merino DM, McShane L, Butler M et al (2019) TMB standardization by alignment to reference standards: phase II of the friends of cancer research TMB harmonization project. J Clin Oncol 37:2624–2624. https://doi.org/10.1200/jco.2019.37.15_suppl.2624
Chan TA, Yarchoan M, Jaffee E et al (2019) Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 30:44–56. https://doi.org/10.1093/ANNONC/MDY495
Havel JJ, Chowell D, Chan TA (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19:133–150
Van Allen EM, Miao D, Schilling B et al (2015) Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350:207–211. https://doi.org/10.1126/science.aad0095
Rizvi NA, Hellmann MD, Snyder A et al (2015) Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128. https://doi.org/10.1126/science.aaa1348
Yarchoan M, Hopkins A, Jaffee EM (2017) Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377:2500–2501. https://doi.org/10.1056/NEJMc1713444
Marabelle A, Fakih M, Lopez J et al (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21:1353–1365. https://doi.org/10.1016/S1470-2045(20)30445-9
O’Meara TA, Tolaney SM (2021) Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget 12:394–400
Barroso-Sousa R, Jain E, Cohen O et al (2020) Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol 31:387–394. https://doi.org/10.1016/j.annonc.2019.11.010
Alva AS, Mangat PK, Garrett-Mayer E et al (2021) Pembrolizumab in patients with metastatic breast cancer with high tumor mutational burden: results from the targeted agent and profiling utilization registry (TAPUR) study. J Clin Oncol 39:2443–2451. https://doi.org/10.1200/JCO.20.02923
Winer EP, Lipatov O, Im S-A et al (2020) Association of tumor mutational burden (TMB) and clinical outcomes with pembrolizumab (pembro) versus chemotherapy (chemo) in patients with metastatic triple-negative breast cancer (mTNBC) from KEYNOTE-119. J Clin Oncol 38:1013–1013. https://doi.org/10.1200/jco.2020.38.15_suppl.1013
Karn T, Denkert C, Weber KE et al (2020) Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann Oncol 31:1216–1222. https://doi.org/10.1016/j.annonc.2020.05.015
Barroso-Sousa R, Trippa L, Lange P et al (2019) Nimbus: a phase II study of nivolumab plus ipilimumab in metastatic hypermutated HER2-negative breast cancer. J Clin Oncol 37:TPS1115–TPS1115. https://doi.org/10.1200/JCO.2019.37.15_SUPPL.TPS1115
McGrail DJ, Pilié PG, Rashid NU et al (2021) High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol 32:661–672. https://doi.org/10.1016/j.annonc.2021.02.006
Jiricny J (2006) (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 75(7):335–346. https://doi.org/10.1038/NRM1907
Bonneville R, Krook MA, Kautto EA et al (2017) Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. https://doi.org/10.1200/po.17.00073
Vieira MLC, Santini L, Diniz AL, de Munhoz CF (2016) Microsatellite markers: what they mean and why they are so useful. Genet Mol Biol 39:312. https://doi.org/10.1590/1678-4685-GMB-2016-0027
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520. https://doi.org/10.1056/nejmoa1500596
Prasad V, Kaestner V, Mailankody S (2018) Cancer drugs approved based on biomarkers and not tumor type—FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol 4:157–158. https://doi.org/10.1001/JAMAONCOL.2017.4182
Andre T, Berton D, Curigliano G et al (2021) Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J Clin Oncol 39:9–9. https://doi.org/10.1200/JCO.2021.39.3_SUPPL.9
Cheng AS, Leung SCY, Gao D et al (2020) Mismatch repair protein loss in breast cancer: clinicopathological associations in a large British Columbia cohort. Breast Cancer Res Treat 179:3–10. https://doi.org/10.1007/s10549-019-05438-y
Haricharan S, Bainbridge MN, Scheet P, Brown PH (2014) Somatic mutation load of estrogen receptor-positive breast tumors predicts overall survival: an analysis of genome sequence data. Breast Cancer Res Treat 146:211–220. https://doi.org/10.1007/s10549-014-2991-x
Kok M, Horlings HM, Snaebjornsson P et al (2017) Profound immunotherapy response in mismatch repair-deficient breast cancer. JCO Precis Oncol. https://doi.org/10.1200/po.17.00052
Fremd C, Hlevnjak M, Zapatka M et al (2019) Mismatch repair deficiency drives durable complete remission by targeting programmed death receptor 1 in a metastatic luminal breast cancer patient. Breast Care 14:53–59. https://doi.org/10.1159/000492580
Li A, Goodyear S, Fuss C, Mitri Z (2021) Exceptional response to pembrolizumab and trastuzumab in a heavily pretreated patient with HER2-positive TMB-H and MSI-H metastatic breast cancer. JCO Precis Oncol 5:904–909. https://doi.org/10.1200/PO.20.00361
Wang S, Jia M, He Z, Liu XS (2018) APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene 37:3924–3936. https://doi.org/10.1038/s41388-018-0245-9
Roberts SA, Lawrence MS, Klimczak LJ et al (2013) An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet 45:970–976. https://doi.org/10.1038/ng.2702
Lefebvre C, Bachelot T, Filleron T et al (2016) Mutational profile of metastatic breast cancers: a retrospective analysis. PLOS Med 13:e1002201. https://doi.org/10.1371/journal.pmed.1002201
Chumsri S, Sokol ES, Soyano-Muller AE et al (2020) Durable complete response with immune checkpoint inhibitor in breast cancer with high tumor mutational burden and APOBEC signature. JNCCN J Natl Compr Cancer Netw 18:517–521. https://doi.org/10.6004/jnccn.2020.7543
Dimarco AV, Qin X, Van Alsten S et al (2021) APOBEC mutagenesis inhibits breast cancer growth through induction of a T cell-mediated antitumor immune response. bioRxiv. https://doi.org/10.1101/2021.02.13.431068
Bertucci F, Ng CKY, Patsouris A et al (2019) Genomic characterization of metastatic breast cancers. Nature 569:560–564. https://doi.org/10.1038/s41586-019-1056-z
Mao Y, Lv M, Zhang Y et al (2020) APOBEC3B expression and its prognostic potential in breast cancer. Oncol Lett 19:3205–3214. https://doi.org/10.3892/ol.2020.11433
Kanu N, Cerone MA, Goh G et al (2016) DNA replication stress mediates APOBEC3 family mutagenesis in breast cancer. Genome Biol. https://doi.org/10.1186/s13059-016-1042-9
Huang RSP, Haberberger J, Severson E et al (2020) A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod Pathol 342(34):252–263. https://doi.org/10.1038/s41379-020-00664-y
Goodman AM, Piccioni D, Kato S et al (2018) Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol 4:1237–1244. https://doi.org/10.1001/JAMAONCOL.2018.1701
Gupta S, Vanderbilt CM, Cotzia P et al (2019) Next-generation sequencing-based assessment of JAK2, PD-L1, and PD-L2 copy number alterations at 9p24.1 in breast cancer: potential implications for clinical management. J Mol Diagn 21:307–317. https://doi.org/10.1016/J.JMOLDX.2018.10.006
Bachelot T, Filleron T, Dalenc F et al (2020) 128O PDL1/CD274 gain/amplification as a predictive marker of checkpoint blockade inhibitor efficacy in metastatic breast cancer: Exploratory analysis of the SAFIR02-IMMUNO randomized phase II trial. Ann Oncol 31:S58–S59. https://doi.org/10.1016/J.ANNONC.2020.03.231
Rayner E, van Gool IC, Palles C et al (2016) A panoply of errors: polymerase proofreading domain mutations in cancer. Nat Rev Cancer 162(16):71–81. https://doi.org/10.1038/nrc.2015.12
Wang F, Zhao Q, Wang Y-N et al (2019) Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol 5:1504–1506. https://doi.org/10.1001/JAMAONCOL.2019.2963
Mittica G, Ghisoni E, Giannone G et al (2017) Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity. Oncotarget 8:90532–90544. https://doi.org/10.18632/ONCOTARGET.20042
Voutsadakis IA (2019) High tumor mutation burden and other immunotherapy response predictors in breast cancers: associations and therapeutic opportunities. Target Oncol 151(15):127–138. https://doi.org/10.1007/S11523-019-00689-7
Ayers M, Lunceford J, Nebozhyn M et al (2017) IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 127:2930–2940. https://doi.org/10.1172/JCI91190
Damotte D, Warren S, Arrondeau J et al (2019) The tumor inflammation signature (TIS) is associated with anti-PD-1 treatment benefit in the CERTIM pan-cancer cohort. J Transl Med 17:357. https://doi.org/10.1186/s12967-019-2100-3
Danaher P, Warren S, Lu R et al (2018) Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from the cancer genome atlas (TCGA). J Immunother Cancer. https://doi.org/10.1186/s40425-018-0367-1
Ciruelos E, Pascual T, Chic N, et al (2021) Abstract OT-13-04: Solti-1716. Targeting non-luminal disease by PAM50 with pembrolizumab + paclitaxel in hormone receptor-positive/HER2-negative advanced/metastatic breast cancer patients who have progressed on or after CDK 4/6 inhibitor treatment (TATEN). In: Cancer Research. American Association for Cancer Research (AACR), p OT-13-04-OT-13-04
Brasó-Maristany F, Paré L, Chic N et al (2021) Gene expression profiles of breast cancer metastasis according to organ site. Mol Oncol 1878–0261:13021. https://doi.org/10.1002/1878-0261.13021
Cristescu R, Mogg R, Ayers M et al (2018) Pan-tumor genomic biomarkers for PD-1 checkpoint blockade–based immunotherapy. Science 362:3593. https://doi.org/10.1126/SCIENCE.AAR3593
Paré L, Pascual T, Seguí E et al (2018) Association between PD1 mRNA and response to anti-PD1 monotherapy across multiple cancer types. Ann Oncol 29:2121–2128. https://doi.org/10.1093/annonc/mdy335
Emens LA, Goldstein LD, Schmid P et al (2021) The tumor microenvironment (TME) and atezolizumab + nab-paclitaxel (A+nP) activity in metastatic triple-negative breast cancer (mTNBC): IMpassion130. J Clin Oncol 39:1006–1006. https://doi.org/10.1200/JCO.2021.39.15_SUPPL.1006
Burstein MD, Tsimelzon A, Poage GM et al (2015) Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 21:1688. https://doi.org/10.1158/1078-0432.CCR-14-0432
Bianchini G, Dugo M, Huang C-S et al (2021) LBA12 predictive value of gene-expression profiles (GEPs) and their dynamics during therapy in the NeoTRIPaPDL1 trial. Ann Oncol 32:S1283–S1284. https://doi.org/10.1016/J.ANNONC.2021.08.2084
Dieci MV, Griguolo G, Bisagni G et al (2021) 129P Integration of gene expression and tumor-infiltrating lymphocytes (TILs) to predict pCR after neoadjuvant chemotherapy and nivolumab for patients with luminal B-like breast cancer in the phase II GIADA trial. Ann Oncol 32:S414. https://doi.org/10.1016/J.ANNONC.2021.08.410
Wan JCM, Massie C, Garcia-Corbacho J et al (2017) Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 17:223–238. https://doi.org/10.1038/nrc.2017.7
Cabel L, Proudhon C, Romano E et al (2018) Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol 1510(15):639–650. https://doi.org/10.1038/S41571-018-0074-3
Lee JH, Long GV, Boyd S et al (2017) Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol 28:1130–1136. https://doi.org/10.1093/annonc/mdx026
Powles T, Assaf ZJ, Davarpanah N et al (2021) ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 5957867(595):432–437. https://doi.org/10.1038/S41586-021-03642-9
Georgiadis A, Durham JN, Keefer LA et al (2019) Noninvasive detection of microsatellite instability and high tumor mutation burden in cancer patients treated with PD-1 blockade. Clin Cancer Res 25:7024–7034. https://doi.org/10.1158/1078-0432.CCR-19-1372
Gandara DR, Kowanetz M, Mok TSK, et al (2017) Blood-based biomarkers for cancer immunotherapy: tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L1 NSCLC (POPLAR and OAK). Abstr B 42nd ESMO Congr (ESMO 2017) 8–12 Sept 2017, Madrid, Spain 28:v460. https://doi.org/10.1093/annonc/mdx380
Rizvi NA, Cho BC, Reinmuth N et al (2020) Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 6:661–674. https://doi.org/10.1001/jamaoncol.2020.0237
Bratman SV, Yang SYC, Iafolla MAJ et al (2020) Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer 1:873–881. https://doi.org/10.1038/s43018-020-0096-5
Zhang Q, Luo J, Wu S et al (2020) Prognostic and predictive impact of circulating tumor DNA in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discov. https://doi.org/10.1158/2159-8290.cd-20-0047
Hieken T, ChenJ HT et al (2016) The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. https://doi.org/10.1038/SREP30751
Wang H, Altemus J, Niazi F et al (2017) Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget 8:88122–88138. https://doi.org/10.18632/ONCOTARGET.21490
Xuan C, Shamonki J, Chung A et al (2014) Microbial dysbiosis is associated with human breast cancer. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0083744
Gopalakrishnan V, Spencer CN, Nezi L et al (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359:97–103. https://doi.org/10.1126/SCIENCE.AAN4236
Sivan A, Corrales L, Hubert N et al (2015) Commensal bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 350:1084. https://doi.org/10.1126/SCIENCE.AAC4255
Blank CU, Haanen JB, Ribas A, Schumacher TN (2016) The “cancer immunogram.” Science 352:658–660. https://doi.org/10.1126/SCIENCE.AAF2834
Acknowledgements
This study has received funding from Generalitat de Catalunya Peris PhD4MD 2019 SLT008/18/00122 (to N.C.), Fundación Científica Asociación Española Contra el Cáncer AECC_Postdoctoral17-1062 (to F.B-M.).
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.P. reports advisory and consulting fees from Roche, Pfizer, Novartis, Amgen, BMS, Puma, Oncolytics Biotech, MSD, Guardant Health, Peptomyc and Lilly, lecture fees from Roche, Pfizer, Novartis, Amgen, BMS, Nanostring Technologies and Daiichi Sankyo, institutional financial interests from Boehringer, Novartis, Roche, Nanostring, Sysmex Europa GmbH, Medica Scientia inno. Research, SL, Celgene, Astellas and Pfizer; a leadership role in Reveal Genomics, SL; and a patent PCT/EP2016/080056.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chic, N., Brasó-Maristany, F. & Prat, A. Biomarkers of immunotherapy response in breast cancer beyond PD-L1. Breast Cancer Res Treat 191, 39–49 (2022). https://doi.org/10.1007/s10549-021-06421-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06421-2