Abstract
Purpose
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer that is frequently treated with chemotherapy. However, many patients exhibit either de novo chemoresistance or ultimately develop resistance to chemotherapy, leading to significantly high mortality rates. Therefore, increasing the efficacy of chemotherapy has potential to improve patient outcomes.
Methods
Here, we performed whole transcriptome sequencing (both RNA and small RNA-sequencing), coupled with network simulations and patient survival data analyses to build a novel miRNA-mRNA interaction network governing chemoresistance in TNBC. We performed cell proliferation assay, Western blotting, RNAi/miRNA mimic experiments, FN coating, 3D cultures, and ChIP assays to validate the interactions in the network, and their functional roles in chemoresistance. We developed xenograft models to test the therapeutic potential of the identified key miRNA/proteins in potentiating chemoresponse in vivo. We also analyzed several patient datasets to evaluate the clinical relevance of our findings.
Results
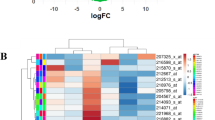
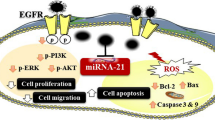
We identified fibronectin (FN1) as a central chemoresistance driver gene. Overexpressing miR-326 reversed FN1-driven chemoresistance by targeting FN1 receptor, ITGA5. miR-326 was downregulated by increased hypoxia/HIF1A and ECM stiffness in chemoresistant tumors, leading to upregulation of ITGA5 and activation of the downstream FAK/Src signaling pathways. Overexpression of miR-326 or inhibition of ITGA5 overcame FN1-driven chemotherapy resistance in vitro by inhibiting FAK/Src pathway and potentiated the efficacy of chemotherapy in vivo. Importantly, lower expression of miR-326 or higher levels of predicted miR-326 target genes was significantly associated with worse overall survival in chemotherapy-treated TNBC patients.
Conclusion
FN1 is central in chemoresistance. In chemoresistant tumors, hypoxia and resulting ECM stiffness repress the expression of the tumor suppressor miRNA, miR-326. Hence, re-expression of miR-326 or inhibition of its target ITGA5 reverses FN1-driven chemoresistance making them attractive therapeutic approaches to enhance chemotherapy response in TNBCs.






Similar content being viewed by others
Data availability
Raw RNA-seq data analyzed in the current study have been made available on NCBI Sequence Read Archive with accession PRJNA607780. The small RNA-Seq data have also been deposited to the SRA with the submission ID: PRJNA806717.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Hwang SY, Park S, Kwon Y (2019) Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol Ther 199:30–57. https://doi.org/10.1016/j.pharmthera.2019.02.006
Boyle P (2012) Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 23(6):7–12. https://doi.org/10.1093/annonc/mds187
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13:674–690. https://doi.org/10.1038/nrclinonc.2016.66
Santonja A, Sanchez-Munoz A, Lluch A, Chica-Parrado MR, Albanell J, Chacon JI et al (2018) Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 9:26406–26416. https://doi.org/10.18632/oncotarget.25413
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F et al (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329–2334. https://doi.org/10.1158/1078-0432.CCR-06-1109
Gass P, Lux MP, Rauh C, Hein A, Bani MR, Fiessler C et al (2018) Prediction of pathological complete response and prognosis in patients with neoadjuvant treatment for triple-negative breast cancer. BMC Cancer 18:1051. https://doi.org/10.1186/s12885-018-4925-1
Lebert JM, Lester R, Powell E, Seal M, McCarthy J (2018) Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol 25:S142–S150. https://doi.org/10.3747/co.25.3954
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281. https://doi.org/10.1200/JCO.2007.14.4147
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Keenan TE, Tolaney SM (2020) Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw 18:479–489. https://doi.org/10.6004/jnccn.2020.7554
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR et al (2019) Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 380:741–751. https://doi.org/10.1056/NEJMoa1814213
Egeblad M, Nakasone ES, Werb Z (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18:884–901. https://doi.org/10.1016/j.devcel.2010.05.012
Henke E, Nandigama R, Ergun S (2019) Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 6:160. https://doi.org/10.3389/fmolb.2019.00160
Saatci O, Kaymak A, Raza U, Ersan PG, Akbulut O, Banister CE et al (2020) Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun 11:2416. https://doi.org/10.1038/s41467-020-16199-4
Berrazouane S, Boisvert M, Salti S, Mourad W, Al-Daccak R, Barabe F et al (2019) Beta1 integrin blockade overcomes doxorubicin resistance in human T-cell acute lymphoblastic leukemia. Cell Death Dis 10:357. https://doi.org/10.1038/s41419-019-1593-2
Cooper J, Giancotti FG (2019) Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 35:347–367. https://doi.org/10.1016/j.ccell.2019.01.007
Lovitt CJ, Shelper TB, Avery VM (2018) Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 18:41. https://doi.org/10.1186/s12885-017-3953-6
Zhu H, Wang G, Zhu H, Xu A (2021) ITGA5 is a prognostic biomarker and correlated with immune infiltration in gastrointestinal tumors. BMC Cancer 21:269. https://doi.org/10.1186/s12885-021-07996-1
Zhou C, Shen Y, Wei Z, Shen Z, Tang M, Shen Y et al (2022) ITGA5 is an independent prognostic biomarker and potential therapeutic target for laryngeal squamous cell carcinoma. J Clin Lab Anal. https://doi.org/10.1002/jcla.24228
Gilbert LA, Hemann MT (2011) Chemotherapeutic resistance: surviving stressful situations. Cancer Res 71:5062–5066. https://doi.org/10.1158/0008-5472.CAN-11-0277
Senthebane DA, Rowe A, Thomford NE, Shipanga H, Munro D, Mazeedi M et al (2017) The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci. https://doi.org/10.3390/ijms18071586
Hwang D, Rust AG, Ramsey S, Smith JJ, Leslie DM, Weston AD et al (2005) A data integration methodology for systems biology. Proc Natl Acad Sci U S A 102:17296–17301. https://doi.org/10.1073/pnas.0508647102
Archer TC, Fertig EJ, Gosline SJ, Hafner M, Hughes SK, Joughin BA et al (2016) Systems approaches to cancer biology. Cancer Res 76:6774–6777. https://doi.org/10.1158/0008-5472.CAN-16-1580
Nagy A, Lanczky A, Menyhart O, Gyorffy B (2018) Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep 8:9227. https://doi.org/10.1038/s41598-018-27521-y
Jezequel P, Loussouarn D, Guerin-Charbonnel C, Campion L, Vanier A, Gouraud W et al (2015) Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res 17:43. https://doi.org/10.1186/s13058-015-0550-y
Zhao XG, Hu JY, Tang J, Yi W, Zhang MY, Deng R et al (2019) miR-665 expression predicts poor survival and promotes tumor metastasis by targeting NR4A3 in breast cancer. Cell Death Dis 10:479. https://doi.org/10.1038/s41419-019-1705-z
Enerly E, Steinfeld I, Kleivi K, Leivonen SK, Aure MR, Russnes HG et al (2011) miRNA-mRNA integrated analysis reveals roles for miRNAs in primary breast tumors. PLoS ONE 6:e16915. https://doi.org/10.1371/journal.pone.0016915
de Rinaldis E, Gazinska P, Mera A, Modrusan Z, Fedorowicz GM, Burford B et al (2013) Integrated genomic analysis of triple-negative breast cancers reveals novel microRNAs associated with clinical and molecular phenotypes and sheds light on the pathways they control. BMC Genomics 14:643. https://doi.org/10.1186/1471-2164-14-643
Agarwal V, Bell GW, Nam JW, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. Elife. https://doi.org/10.7554/eLife.05005
Szot CS, Buchanan CF, Freeman JW, Rylander MN (2011) 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 32:7905–7912. https://doi.org/10.1016/j.biomaterials.2011.07.001
Motte S, Kaufman LJ (2013) Strain stiffening in collagen I networks. Biopolymers 99:35–46. https://doi.org/10.1002/bip.22133
Anguiano M, Castilla C, Maska M, Ederra C, Pelaez R, Morales X et al (2017) Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis. PLoS ONE 12:e0171417. https://doi.org/10.1371/journal.pone.0171417
Kimura H, Weisz A, Ogura T, Hitomi Y, Kurashima Y, Hashimoto K et al (2001) Identification of hypoxia-inducible factor 1 ancillary sequence and its function in vascular endothelial growth factor gene induction by hypoxia and nitric oxide. J Biol Chem 276:2292–2298. https://doi.org/10.1074/jbc.M008398200
Rashid I, Pathak AK, Kumar R, Srivastava P, Singh M, Murali S et al (2019) Genome-wide comparative analysis of HIF binding sites in cyprinus carpio for in silico identification of functional hypoxia response elements. Front Genet 10:659. https://doi.org/10.3389/fgene.2019.00659
Singh P, Carraher C, Schwarzbauer JE (2010) Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26:397–419. https://doi.org/10.1146/annurev-cellbio-100109-104020
Hamidi H, Pietila M, Ivaska J (2016) The complexity of integrins in cancer and new scopes for therapeutic targeting. Br J Cancer 115:1017–1023. https://doi.org/10.1038/bjc.2016.312
Ju JA, Godet I, Ye IC, Byun J, Jayatilaka H, Lee SJ et al (2017) Hypoxia selectively enhances integrin alpha5beta1 receptor expression in breast cancer to promote metastasis. Mol Cancer Res 15:723–734. https://doi.org/10.1158/1541-7786.MCR-16-0338
Schaffner F, Ray AM, Dontenwill M (2013) Integrin alpha5beta1, the fibronectin receptor, as a pertinent therapeutic target in solid tumors. Cancers 5:27–47. https://doi.org/10.3390/cancers5010027
Morozevich GE, Kozlova NI, Susova OY, Lupatov AY, Berman AE (2017) Hyperexpression of Integrin alpha5beta1 Promotes Resistance of MCF-7 Human Breast Carcinoma Cells to Doxorubicin via ERK Protein Kinase Down-regulation. Biochemistry (Mosc) 82:1017–1024. https://doi.org/10.1134/S0006297917090048
Aota S, Nomizu M, Yamada KM (1994) The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem 269:24756–24761
Chavez KJ, Garimella SV, Lipkowitz S (2010) Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis 32:35–48. https://doi.org/10.3233/BD-2010-0307
Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res 13:215. https://doi.org/10.1186/bcr2889
Lucero HA, Kagan HM (2006) Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63:2304–2316. https://doi.org/10.1007/s00018-006-6149-9
Ghaemi Z, Soltani BM, Mowla SJ (2019) MicroRNA-326 functions as a tumor suppressor in breast cancer by targeting ErbB/PI3K signaling pathway. Front Oncol 9:653. https://doi.org/10.3389/fonc.2019.00653
Hu S, Ran Y, Chen W, Zhang Y, Xu Y (2017) MicroRNA-326 inhibits cell proliferation and invasion, activating apoptosis in hepatocellular carcinoma by directly targeting LIM and SH3 protein 1. Oncol Rep 38:1569–1578. https://doi.org/10.3892/or.2017.5810
Hong BS, Ryu HS, Kim N, Kim J, Lee E, Moon H et al (2019) Tumor suppressor miRNA-204-5p regulates growth, metastasis, and immune microenvironment remodeling in breast cancer. Cancer Res 79:1520–1534. https://doi.org/10.1158/0008-5472.CAN-18-0891
Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK (2016) miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov 6:235–246. https://doi.org/10.1158/2159-8290.CD-15-0893
Zhang P, Kong F, Deng X, Yu Y, Hou C, Liang T et al (2017) MicroRNA-326 suppresses the proliferation, migration and invasion of cervical cancer cells by targeting ELK1. Oncol Lett 13:2949–2956. https://doi.org/10.3892/ol.2017.5852
Sun C, Huang C, Li S, Yang C, Xi Y, Wang L et al (2016) Hsa-miR-326 targets CCND1 and inhibits non-small cell lung cancer development. Oncotarget 7:8341–8359. https://doi.org/10.18632/oncotarget.7071
Nawaz Z, Patil V, Paul Y, Hegde AS, Arivazhagan A, Santosh V et al (2016) PI3 kinase pathway regulated miRNome in glioblastoma: identification of miR-326 as a tumour suppressor miRNA. Mol Cancer 15:74. https://doi.org/10.1186/s12943-016-0557-8
Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K et al (2010) Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol 79:817–824. https://doi.org/10.1016/j.bcp.2009.10.017
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266–276. https://doi.org/10.1093/jnci/93.4.266
Rohwer N, Cramer T (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 14:191–201. https://doi.org/10.1016/j.drup.2011.03.001
Bertout JA, Patel SA, Simon MC (2008) The impact of O2 availability on human cancer. Nat Rev Cancer 8:967–975. https://doi.org/10.1038/nrc2540
Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G et al (2003) Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res 63:1138–1143
Song X, Liu X, Chi W, Liu Y, Wei L, Wang X et al (2006) Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol 58:776–784. https://doi.org/10.1007/s00280-006-0224-7
Botla SK, Gholami AM, Malekpour M, Moskalev EA, Fallah M, Jandaghi P et al (2012) Diagnostic values of GHSR DNA methylation pattern in breast cancer. Breast Cancer Res Treat 135:705–713. https://doi.org/10.1007/s10549-012-2197-z
Mishra RR, Belder N, Ansari SA, Kayhan M, Bal H, Raza U et al (2018) Reactivation of cAMP pathway by PDE4D inhibition represents a novel druggable axis for overcoming tamoxifen resistance in ER-positive breast cancer. Clin Cancer Res 24:1987–2001. https://doi.org/10.1158/1078-0432.CCR-17-2776
Saatci O, Borgoni S, Akbulut O, Durmus S, Raza U, Eyupoglu E et al (2018) Targeting PLK1 overcomes T-DM1 resistance via CDK1-dependent phosphorylation and inactivation of Bcl-2/xL in HER2-positive breast cancer. Oncogene 37:2251–2269. https://doi.org/10.1038/s41388-017-0108-9
Raza U, Saatci O, Uhlmann S, Ansari SA, Eyupoglu E, Yurdusev E et al (2016) The miR-644a/CTBP1/p53 axis suppresses drug resistance by simultaneous inhibition of cell survival and epithelial-mesenchymal transition in breast cancer. Oncotarget 7:49859–49877. https://doi.org/10.18632/oncotarget.10489
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q et al (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 123:725–731. https://doi.org/10.1007/s10549-009-0674-9
Dweep H, Gretz N (2015) miRWalk20: a comprehensive atlas of microRNA-target interactions. Nat Methods 12:697. https://doi.org/10.1038/nmeth.3485
Acknowledgements
We are thankful to the members of the Ozgur Sahin laboratory for invaluable discussion and advice. We thank Jitao David Zhang for his help with RNA-sequencing data.
Funding
This work was supported by European Commission FP7 Marie Curie Career Integration Grant PCIG14-GA-2013-631149 (OS), the American Cancer Society Institutional Research Grant IRG-17-179-04 (OS), NIH Research Project Grants R01-CA267101 (OS) and 2P20GM109091-06 (OS), the scholarship from Higher Education Commission of Pakistan (UR), the scholarship from 2211/A TÜBİTAK Domestic PhD Scholarship Program (UMT), and Susan G. Komen Interdisciplinary Graduate Training to Eliminate Cancer Disparities (IGniTE-CD) GTDR17500160 (OzgeS). YR is a research scholar of Fonds de recherche du Québec—Santé (FRQS).
Author information
Authors and Affiliations
Contributions
OS supervised the study. OS and UR conceptualized the study. RA, UMT, IOT, OzgeS, PG, and UR designed and performed the experiments, data acquisition, and data analysis. RA, UMT, IOT, OzgeS, and OS contributed to writing, review, and/or revision of the manuscript. HO and TC helped building the miRNA–mRNA network. YR oversaw RNA-seq and small RNA-seq experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
O.S. is the co-founder and manager of OncoCube Therapeutics LLC and founder and president of LoxiGen, Inc. The other authors declare no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Assidicky, R., Tokat, U.M., Tarman, I.O. et al. Targeting HIF1-alpha/miR-326/ITGA5 axis potentiates chemotherapy response in triple-negative breast cancer. Breast Cancer Res Treat 193, 331–348 (2022). https://doi.org/10.1007/s10549-022-06569-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06569-5