Abstract
Purpose
Studies have controversially suggested that prostate cancer, the most common cancer among Western men, is less common among those with a high intake of tomato products and lycopene. We examine multivariable associations between the intake of tomatoes and lycopene, and risk of prostate cancer.
Methods
In a prospective study of 27,934 Adventist men without prevalent cancer, Cox proportional hazard regression analyses were used to address the objectives. Dietary measurement error was partially corrected with regression calibration.
Results
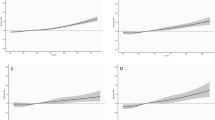
1226 incident cases of prostate cancer, 355 of them aggressive, were identified during 7.9 years of follow-up. Consumption of canned and cooked tomatoes more than four times a week was associated with a HR = 0.72 (95% CI 0.55, 0.94, P = 0.02) comparing to risk in those never consuming this food. Treating this as a continuous variable, adjusting for confounders, produces a similar result, HR = 0.86 (95% CI 0.75, 0.99), comparing 64 g/day with zero intakes (questionnaire data). Regression calibration, although less precise, suggests a yet stronger and statistically significant inverse relationship, comparing a 24-h dietary recall intake of 71 g/day canned and cooked tomato product, with zero intake. Uncalibrated multivariable-adjusted competing risk analyses do not find differences in tomato associations between aggressive and non-aggressive prostate cancers although power for aggressive cancers is limited.
Conclusion
Consumption of canned and cooked tomatoes may reduce the risk of prostate cancer. These products contain more available lycopene. However, an observational study cannot exclude confounding by some unidentified, prostate cancer preventive factor.
Clinical Trial Registry: ClinicalTrials.gov Identifier: NCT03615599

Similar content being viewed by others
Abbreviations
- PSA:
-
Prostate-specific antigen
- AICR/WCRF:
-
The American Institute for Cancer Research/World Cancer Research Fund
- AHS-1:
-
The Adventist Health Study-1
- AHS-2:
-
The Adventist Health Study-2
- NDSR:
-
Nutrition data system for research software versions 4, and 5
- NCC:
-
Nutrition Coordinating Center
- ICD-10:
-
International classification of diseases-10
- SEER:
-
Surveillance epidemiology and end results
- BPH:
-
Benign prostate hypertrophy
References
Center MM, Jemal A, Lortet-Tieulent J et al (2012) International variation in prostate cancer incidence and mortality rates. Eur Urol 61:1079–1092
American Cancer Society (2018) Cancer facts & figures 2018. American Cancer Society, Atlanta
Zlotta AR, Egawa S, Pushkar D et al (2013) Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 105:1050–1058
Watanabe M, Nakayama T, Shiraishi T et al (2000) Comparative studies of prostate cancer in Japan versus the United States. A review. Urol Oncol 5:274–283
Discacciati A, Orsini N, Wolk A (2012) Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol 23:1665–1671
Blot WJ, Tarone RE (2015) Doll and Peto's quantitative estimates of cancer risks: holding generally true for 35 years. J Natl Cancer Inst 107(4):djv044
Doll R, Peto R (1981) The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 66:1191–1308
World Cancer Research Fund International (2014) Continuous update project report: diet, nutrition, physical activity, and prostate cancer. World Cancer Research Fund International, https://wwwwcrf.org/sites/default/files/Prostate-Cancer-2014Report.pdf
Xu X, Li J, Wang X et al (2016) Tomato consumption and prostate cancer risk: a systematic review and meta-analysis. Sci Rep 6:37091
Chen J, Song Y, Zhang L (2013) Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta-analysis of prospective studies. J Nutr Sci Vitaminol 59:213–223
Holzapfel NP, Holzapfel BM, Champ S et al (2013) The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci 14:14620–14646
Lin PH, Aronson W, Freedland SJ (2015) Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC Med 13:3
Giovannucci E, Rimm EB, Liu Y et al (2002) A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst 94:391–398
Rowles III JL, Ranard KM, Applegate CC et al (2018) Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis. Prostate Cancer Prostatic Dis 21:319–336
Wang Y, Cui R, Xiao Y et al (2015) Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose-response meta-analysis of observational studies. PLoS ONE 10:e0137427
Wang Y, Cui R, Xiao Y et al (2015) Correction: effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose-response meta-analysis of observational studies. PLoS ONE 10:e0140415
Chen P, Zhang W, Wang X et al (2015) Lycopene and risk of prostate cancer: a systematic review and meta-analysis. Medicine 94:e1260
Rowles III JL, Ranard KM, Smith JW et al (2017) Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 20:361–377
Gathirua-Mwangi WG, Zhang J (2014) Dietary factors and risk for advanced prostate cancer. Eur J Cancer Prev 23:96–109
Zu K, Mucci L, Rosner BA, et al. (2014) Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst 106:djt430
Mills PK, Beeson WL, Phillips RL et al (1989) Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 64:598–604
Wei MY, Giovannucci EL (2012) Lycopene, tomato products, and prostate cancer incidence: a review and reassessment in the PSA Screening Era. J Oncol 2012:271063
Butler TL, Fraser GE, Beeson WL et al (2008) Cohort profile: The Adventist Health Study-2 (AHS-2). Int J Epidemiol 37:260–265
Jaceldo-Siegl K, Knutsen SF, Sabate J et al (2010) Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr 13:812–819
Jaceldo-Siegl K, Fan J, Sabate J et al (2011) Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr 14:1988–1997
Schakel SF (2001) Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products—a research perspective. J Food Compos Anal 14:315–322
Orlich MJ, Singh PN, Sabate J et al (2013) Vegetarian dietary patterns and mortality in Adventist Health Study 2. Jama Intern Med 173:1230–1238
World Health Organization (2000) Obesity: preventing and managing the global epidemic Report of a WHO Consultation (WHO Technical Report Series 894). WHO, Geneva, p 252
Tantamango-Bartley Y, Knutsen SF, Knutsen R et al (2016) Are strict vegetarians protected against prostate cancer? Am J Clin Nutr 103:153–160
Aune D, Navarro Rosenblatt DA, Chan DS et al (2015) Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 101:87–117
Core Team R (2019) A language and environment for statistical computing. R Core Team, Vienna
Fraser G, Yan R (2007) Guided multiple imputation of missing data: using a subsample to strengthen the missing-at-random assumption. Epidemiology 18:246–252
Fraser GE, Stram DO (2012) Regression calibration when foods (measured with error) are the variables of interest: markedly non-Gaussian data with many zeroes. Am J Epidemiol 175:325–331
DiCiccio TJ, Efron B (1996) Bootstrap confidence intervals. Stat Sci 11:189–212
Willett W (1998) Nutritional epidemiology, 2nd edn. Oxford University Press, New York
Xue X, Kim MY, Gaudet MM et al (2013) A comparison of the polytomous logistic regression and joint cox proportional hazards models for evaluating multiple disease subtypes in prospective cohort studies. Cancer Epidemiol Biomarkers Prev 22:275–285
SAS Institute Inc (2017) SAS/STAT ® 14.3 user's Guide. SAS Institute Inc., Cary
Key TJ, Appleby PN, Travis RC et al (2015) Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr 102:1142–1157
Unlu NZ, Bohn T, Francis DM et al (2007) Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br J Nutr 98:140–146
Agarwal A, Shen H, Agarwal S et al (2001) Lycopene content of tomato products: its stability, bioavailability and in vivo antioxidant properties. J Med Food 4:9–15
van Die MD, Bone KM, Emery J et al (2016) Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: a systematic review of randomised trials. BJU Int 117(Suppl 4):17–34
Kim HS, Bowen P, Chen L et al (2003) Effects of tomato sauce consumption on apoptotic cell death in prostate benign hyperplasia and carcinoma. Nutr Cancer 47:40–47
Graff RE, Pettersson A, Lis RT et al (2016) Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr 103:851–860
Falzarano SM, Magi-Galluzzi C (2013) ERG protein expression as a biomarker of prostate cancer. Biomark Med 7:851–865
Drazer MW, Huo D, Eggener SE (2015) National prostate cancer screening rates after the 2012 US preventive services task force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol 33:2416–2423
Kyrgiou M, Kalliala I, Markozannes G et al (2017) Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 356:j477
Powell IJ (2007) Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol 177:444–449
Acknowledgements
The present analyses were supported by grants from the National Cancer Institute (5R01 CA094594) and World Cancer Research Fund Grant (2009/93), to the Adventist Health Study. These funding agencies had no further role in the collection of data, statistical analysis or writing. Cancer incidence data have been provided by the “Alaska Cancer Registry”, “Alberta Health Services”, “Alabama State Cancer Registry”, “Arizona Cancer Registry”, “Arkansas Cancer Registry”, “British Columbia Cancer Agency”, “California Cancer Registry”, “Cancer Care Ontario”, “Colorado Cancer Registry”, “Connecticut Tumor Registry”, “District of Columbia Cancer Registry”, “Delaware Cancer Registry”, “Florida Cancer Data System”, “Georgia Department of Public Health”, “Hawaii Tumor Registry”, “Cancer Data Registry of Idaho (NCI Contract HHSN261201800006I)”, “Iowa Cancer Registry”, “Illinois State Cancer Registry”, “Indiana State Cancer Registry”, “Kansas Cancer Registry”, “Kentucky Cancer Registry”, “Louisiana Tumor Registry”, “Maryland Cancer Registry”, “Massachusetts Cancer Registry”, “Michigan Cancer Surveillance System”, “Minnesota Cancer Surveillance System”, “Mississippi Cancer Registry”, “Missouri Cancer Registry and Research Center”, “Montana Central Tumor Registry”, “Nebraska Cancer Registry”, “Nevada Central Cancer Registry”, “New Hampshire State Cancer Registry”, “New Jersey State Cancer Registry”, “New Mexico Tumor Registry”, “New York State Cancer Registry”, “North Carolina Central Cancer Registry”, “North Dakota Statewide Cancer Registry”, “Cancer Data Registry of Ohio”, “Oklahoma Central Cancer Registry”, “Oregon State Cancer Registry”, “Pennsylvania Cancer Registry”, “Rhode Island Cancer Registry”, “South Carolina Cancer Registry”, “South Dakota Cancer Registry”, “Tennessee Cancer Registry”, “Texas Cancer Registry”, “Utah Cancer Registry, NCI Contract HHSN261201300071”, “Vermont Cancer Registry”, “Virginia Cancer Registry”, “Washington State Cancer Registry”, “West Virginia Cancer Registry”, “Wisconsin Cancer Reporting System”, “Wyoming Cancer Surveillance Program”. The results reported here and the conclusions based on them are the sole responsibility of the authors.
Author information
Authors and Affiliations
Contributions
GEF designed research, conducted research, wrote paper, had primary responsibility for final content; BKJ designed research, wrote paper; SFK conducted research; JIL and AM performed statistical analyses. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fraser, G.E., Jacobsen, B.K., Knutsen, S.F. et al. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: the Adventist Health Study-2. Cancer Causes Control 31, 341–351 (2020). https://doi.org/10.1007/s10552-020-01279-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-020-01279-z