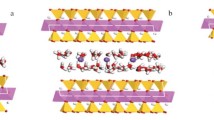
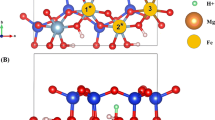
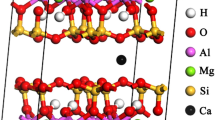
The hydration of clay minerals in shale is one of the main causes of borewall instability. Efficient shale hydration inhibitors require strong interactions between the inhibitor and the mineral surface, such as van der Waals forces, static electricity, hydrogen bonds, and even the formation of chemical bonds, which can significantly reduce the crystal layer spacing of clay minerals. The selection of main functional group of inhibitor plays a decisive role in the performance of inhibitor. The density functional theory method based on quantum mechanics can simulate and calculate the interaction between inhibitor and montmorillonite (001) plane, and study its electronic structure and properties at the atomic level. The adsorption of C2H5–NH2, C2H5–OH, C2H5–OCH3, C2H5–CHO and C2H5–COCH3 on Montmorillonite (001) was calculated by density functional simulation. The adsorption of inhibitor functional groups on montmorillonite (001) layer was studied comprehensively from the aspects of adsorption configuration, adsorption energy, charge population, frontier orbit and differential electron density distribution. According to this study, the primary amine group is suitable as the main functional group of hydration inhibitor. Meanwhile, this paper provides theoretical support for the development of efficient surface hydration inhibitors..















Similar content being viewed by others
References
Gholami R, Elochukwu H, Fakhari N, et al. A review on borehole instability in active shale formations: Interactions, mechanisms and inhibitors[J]. Earth-Science Reviews, 2018, 177: 2-13.
Karpiński B, Szkodo M. Clay minerals–mineralogy and phenomenon of clay swelling in oil & gas industry[J]. Advances in Materials Science, 2015, 15(1): 37-55.
Schoonheydt R A, Johnston C T. Surface and interface chemistry of clay minerals[J]. Developments in clay science, 2006, 1: 87-113.
Hendricks S B, Nelson R A, Alexander L T. Hydration mechanism of the clay mineral montmorillonite saturated with various Cations1[J]. Journal of the American Chemical Society, 1940, 62(6): 1457-1464.
Binqiang X, Chen L, Lihui Z. A Novel Strong Inhibition Water-Based Drilling Fluid Technology[J]. EJGE, 2014, 19: 10499-10510.
Saleh T A. Advanced trends of shale inhibitors for enhanced properties of water-based drilling fluid[J]. Upstream Oil and Gas Technology, 2022, 8: 100069.
Tian Y, Liu X, Luo P, et al. Study of a polyamine inhibitor used for shale water-based drilling fluid[J]. ACS omega, 2021, 6(23): 15448-15459.
Zhao H, Yang Y, Shu X, et al. Adsorption of organic molecules on mineral surfaces studied by first-principle calculations: A review[J]. Advances in Colloid and Interface Science, 2018, 256: 230-241.
Sun X, Liu W, Yang Z, et al. DFT study of the adsorption of 2, 3-epoxypropyltrimethylammonium chloride on montmorillonite surfaces[J]. Journal of Molecular Liquids, 2021, 334: 116145.
Tsuneda T. Density functional theory in quantum chemistry[J]. 2014.
Kryachko E S, Ludena E V. Density functional theory: Foundations reviewed[J]. Physics Reports, 2014, 544(2): 123-239.
Argaman N, Makov G. Density functional theory: An introduction[J]. American Journal of Physics, 2000, 68(1): 69-79.
Swartzen-Allen S L, Matijevic E. Surface and colloid chemistry of clays[J]. Chemical Reviews, 1974, 74(3): 385-400.
Wungu T. D. K., Agusta M. K., Saputro A. G., et al. First principles calculation on the adsorption of water on lithium–montmorillonite (Li–MMT)[J]. Journal of Physics: Condensed Matter, 2012, 24(47): 475506.
Kobayashi K, Yamaguchi A, Okumura M. Machine learning potentials of kaolinite based on the potential energy surfaces of GGA and meta-GGA density functional theory[J]. Applied Clay Science, 2022, 228: 106596.
Yang Z, Liu W, Zhang H, et al. DFT study of the adsorption of 3-chloro-2-hydroxypropyl trimethylammonium chloride on montmorillonite surfaces in solution[J]. Applied Surface Science, 2018, 436: 58-65.
Khoury G A, Gehris T C, Tribe L, et al. Glyphosate adsorption on montmorillonite: An experimental and theoretical study of surface complexes[J]. Applied Clay Science, 2010, 50(2): 167-175.
Liu Y. Is the free energy change of adsorption correctly calculated[J]. Journal of Chemical & Engineering Data, 2009, 54(7): 1981-1985.
Inglezakis V J, Zorpas A A. Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems[J]. Desalination and water treatment, 2012, 39(1-3): 149-157.
Seeman J I. Kenichi Fukui, Frontier Molecular Orbital Theory, and the Woodward-Hoffmann Rules. Part III. Fukui’s Science and Technology, 1918–1965[J]. The Chemical Record, 2022, 22(4): e202100302.
Brush S G. Dynamics of theory change in chemistry: part 2. Benzene and molecular orbitals, 1945–1980[J]. Studies in History and Philosophy of Science Part A, 1999, 30(2): 263-302.
Zollinger H. Color chemistry: syntheses, properties, and applications of organic dyes and pigments[M]. John Wiley & Sons, 2003.
Acknowledgments
The authors cordially acknowledge the financial support of the National Natural Science Foundation of China (No.:51974270) and the Science and Technology Cooperation Project of the CNPC-SWPU Innovation Alliance (No.:2020CX040201). The National Natural Science Foundation: The mechanism and method of reinforcing the reinforced body by plugging in shale water-based drilling fluid (No.:51974270). The Science and Technology Cooperation Project of the CNPC-SWPU Innovation Alliance: Research on matching technology to reduce the complicated conditions and downhole accidents of long horizontal sections (No.:2020CX040201).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 2, pp. 126–133 March– April, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pingquan, W., Tao, T., Junlin, S. et al. Optimization of Main Functional Groups of High Efficiency Hydration Inhibitors in Shale Based on Quantum Mechanical Simulation. Chem Technol Fuels Oils 59, 404–419 (2023). https://doi.org/10.1007/s10553-023-01540-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-023-01540-6