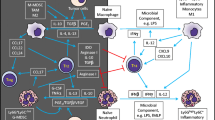
Abstract
The presence of neutrophils in tumors has traditionally been considered to be indicative of a failed immune response against cancers. However, there is now evidence showing that neutrophils can promote tumor growth, and increasingly, the data support an active role for neutrophils in tumor progression to distant metastasis. Neutrophils have been implicated in promoting metastasis in cancer patients, where neutrophil numbers and neutrophil-related factors and functions have been associated with progressive disease. Nevertheless, the role of neutrophils in tumors, both at the primary and secondary sites, remains controversial, with some studies reporting their anti-tumor functions. This review will focus on the data demonstrating a role for neutrophils in both tumor growth and metastasis and will attempt to clarify the discrepancies in the literature.


Similar content being viewed by others
References
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–867.
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454(7203), 436–444.
Pollard, J. W. (2004). Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews Cancer, 4(1), 71–78.
Allavena, P., Sica, A., Solinas, G., Porta, C., & Mantovani, A. (2008). The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical Reviews in Oncology/Hematology, 66(1), 1–9.
Chee, D. O., Townsend, C. M., Jr., Galbraith, M. A., Eilber, F. R., & Morton, D. L. (1978). Selective reduction of human tumor cell populations by human granulocytes in vitro. Cancer Research, 38(12), 4534–4539.
Dvorak, A. M., Connell, A. B., Proppe, K., & Dvorak, H. F. (1978). Immunologic rejection of mammary adenocarcinoma (TA3-St) in C57BL/6 mice: participation of neutrophils and activated macrophages with fibrin formation. Journal of Immunology, 120(4), 1240–1248.
Di Carlo, E., Forni, G., & Musiani, P. (2003). Neutrophils in the antitumoral immune response. Chemical Immunology and Allergy, 83, 182–203.
Souto, J. C., Vila, L., & Bru, A. (2011). Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Medicinal Research Reviews, 31(3), 311–363.
Yan, H. H., Pickup, M., Pang, Y., Gorska, A. E., Li, Z., Chytil, A., et al. (2010). Gr-1 + CD11b + myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Research, 70(15), 6139–6149.
Kaplan, R. N., Riba, R. D., Zacharoulis, S., Bramley, A. H., Vincent, L., Costa, C., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 438(7069), 820–827.
Colotta, F., Re, F., Polentarutti, N., Sozzani, S., & Mantovani, A. (1992). Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood, 80(8), 2012–2020.
Smith, J. A. (1994). Neutrophils, host defense, and inflammation: a double-edged sword. Journal of Leukocyte Biology, 56(6), 672–686.
Ley, K., Laudanna, C., Cybulsky, M. I., & Nourshargh, S. (2007). Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology, 7(9), 678–689.
Hermant, B., Bibert, S., Concord, E., Dublet, B., Weidenhaupt, M., Vernet, T., et al. (2003). Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. Journal of Biological Chemistry, 278(16), 14002–14012.
Nathan, C. (2006). Neutrophils and immunity: challenges and opportunities. Nature Reviews Immunology, 6(3), 173–182.
Mantovani, A., Cassatella, M. A., Costantini, C., & Jaillon, S. (2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology, 11(8), 519–531.
Gabrilovich, D. I., Bronte, V., Chen, S. H., Colombo, M. P., Ochoa, A., Ostrand-Rosenberg, S., et al. (2007). The terminology issue for myeloid-derived suppressor cells. Cancer Research, 67(1), 425. author reply 426.
Bronte, V., Apolloni, E., Cabrelle, A., Ronca, R., Serafini, P., Zamboni, P., et al. (2000). Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood, 96(12), 3838–3846.
Movahedi, K., Guilliams, M., Van den Bossche, J., Van den Bergh, R., Gysemans, C., Beschin, A., et al. (2008). Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood, 111(8), 4233–4244.
Li, H., Han, Y., Guo, Q., Zhang, M., & Cao, X. (2009). Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. Journal of Immunology, 182(1), 240–249.
Rieber, N., Gille, C., Köstlin, N., Schäfer, I., Spring, B., Ost, M., et al. (2013). Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clinical and Experimental Immunology, 174(1), 45–52.
Hoechst, B., Voigtlaender, T., Ormandy, L., Gamrekelashvili, J., Zhao, F., Wedemeyer, H., et al. (2009). Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology, 50(3), 799–807.
Cao, Y., Slaney, C. Y., Bidwell, B. N., Parker, B. S., Johnstone, C. N., Rautela, J., et al. (2014). BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Research, 74(18), 5091–5102.
Serafini, P., Mgebroff, S., Noonan, K., & Borrello, I. (2008). Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Research, 68(13), 5439–5449.
Rodriguez, P. C., Quiceno, D. G., Zabaleta, J., Ortiz, B., Zea, A. H., Piazuelo, M. B., et al. (2004). Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Research, 64(16), 5839–5849.
Rotondo, R., Barisione, G., Mastracci, L., Grossi, F., Orengo, A. M., Costa, R., et al. (2009). IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. International Journal of Cancer, 125(4), 887–893.
Schmielau, J., & Finn, O. J. (2001). Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Research, 61(12), 4756–4760.
Coffelt, S. B., Chen, Y. Y., Muthana, M., Welford, A. F., Tal, A. O., Scholz, A., et al. (2011). Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. Journal of Immunology, 186(7), 4183–4190.
Almand, B., Clark, J. I., Nikitina, E., van Beynen, J., English, N. R., Knight, S. C., et al. (2001). Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. Journal of Immunology, 166(1), 678–689.
Bronte, V., Chappell, D. B., Apolloni, E., Cabrelle, A., Wang, M., Hwu, P., et al. (1999). Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. Journal of Immunology, 162(10), 5728–5737.
Youn, J. I., Kumar, V., Collazo, M., Nefedova, Y., Condamine, T., Cheng, P., et al. (2013). Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nature Immunology, 14(3), 211–220.
Sawanobori, Y., Ueha, S., Kurachi, M., Shimaoka, T., Talmadge, J. E., Abe, J., et al. (2008). Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood, 111(12), 5457–5466.
Youn, J. I., Nagaraj, S., Collazo, M., & Gabrilovich, D. I. (2008). Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of Immunology, 181(8), 5791–5802.
Augier, S., Ciucci, T., Luci, C., Carle, G. F., Blin-Wakkach, C., & Wakkach, A. (2010). Inflammatory blood monocytes contribute to tumor development and represent a privileged target to improve host immunosurveillance. Journal of Immunology, 185(12), 7165–7173.
Fleming, T. J., Fleming, M. L., & Malek, T. R. (1993). Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. Journal of Immunology, 151(5), 2399–2408.
Rose, S., Misharin, A., & Perlman, H. (2012). A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry. Part A, 81(4), 343–350.
Kusmartsev, S., & Gabrilovich, D. I. (2005). STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. Journal of Immunology, 174(8), 4880–4891.
Yang, L., DeBusk, L. M., Fukuda, K., Fingleton, B., Green-Jarvis, B., Shyr, Y., et al. (2004). Expansion of myeloid immune suppressor Gr + CD11b + cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell, 6(4), 409–421.
Peranzoni, E., Zilio, S., Marigo, I., Dolcetti, L., Zanovello, P., Mandruzzato, S., et al. (2010). Myeloid-derived suppressor cell heterogeneity and subset definition. Current Opinion in Immunology, 22(2), 238–244.
Kowanetz, M., Wu, X., Lee, J., Tan, M., Hagenbeek, T., Qu, X., et al. (2010). Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G + Ly6C+ granulocytes. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21248–21255.
Lenzo, J. C., Turner, A. L., Cook, A. D., Vlahos, R., Anderson, G. P., Reynolds, E. C., et al. (2011). Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunology and Cell Biology, 90(4), 429–40.
Movahedi, K., Laoui, D., Gysemans, C., Baeten, M., Stange, G., Van den Bossche, J., et al. (2010). Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C (high) monocytes. Cancer Research, 70(14), 5728–5739.
Granot, Z., Henke, E., Comen, E. A., King, T. A., Norton, L., & Benezra, R. (2011). Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell, 20(3), 300–314.
Bao, Y., & Cao, X. (2011). Revisiting the protective and pathogenic roles of neutrophils: Ly-6G is key! European Journal of Immunology, 41(9), 2535–2538.
Carr, K. D., Sieve, A. N., Indramohan, M., Break, T. J., Lee, S., & Berg, R. E. (2011). Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. European Journal of Immunology, 41(9), 2666–2676.
Bingle, L., Brown, N. J., & Lewis, C. E. (2002). The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. Journal of Pathology, 196(3), 254–265.
Condeelis, J., & Pollard, J. W. (2006). Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell, 124(2), 263–266.
Mantovani, A., Schioppa, T., Porta, C., Allavena, P., & Sica, A. (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer and Metastasis Reviews, 25(3), 315–322.
Kershaw, M. H., Trapani, J. A., & Smyth, M. J. (1995). Cytotoxic lymphocytes: redirecting the cell-mediated immune response for the therapy of cancer. Therapeutic Immunology, 2(3), 173–181.
Ghiringhelli, F., Menard, C., Martin, F., & Zitvogel, L. (2006). The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunology Reviews, 214, 229–238.
Orentas, R. J., Kohler, M. E., & Johnson, B. D. (2006). Suppression of anti-cancer immunity by regulatory T cells: back to the future. Seminars in Cancer Biology, 16(2), 137–149.
Mougiakakos, D., Choudhury, A., Lladser, A., Kiessling, R., & Johansson, C. C. (2010). Regulatory T cells in cancer. Advances in Cancer Research, 107, 57–117.
Draca, S. R. (1993). The participation of natural cytotoxicity in the control of malignant disease. Panminerva Medica, 35(3), 123–126.
Alderson, K. L., & Sondel, P. M. (2011). Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. Journal of Biomedicine and Biotechnology, 2011, 379123.
Gillgrass, A., & Ashkar, A. (2011). Stimulating natural killer cells to protect against cancer: recent developments. Expert Review of Clinical Immunology, 7(3), 367–382.
Johnson, G. R., Whitehead, R., & Nicola, N. A. (1985). Effects of a murine mammary tumor on in vivo and in vitro hemopoiesis. International Journal of Cell Cloning, 3(2), 91–105.
Hardy, C. L., & Balducci, L. (1986). Early hematopoietic events during tumor growth in mice. Journal of the National Cancer Institute, 76(3), 535–540.
Wislez, M., Rabbe, N., Marchal, J., Milleron, B., Crestani, B., Mayaud, C., et al. (2003). Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Research, 63(6), 1405–1412.
Bellocq, A., Antoine, M., Flahault, A., Philippe, C., Crestani, B., Bernaudin, J. F., et al. (1998). Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. American Journal of Pathology, 152(1), 83–92.
Clark, R. A., & Klebanoff, S. J. (1975). Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. Journal of Experimental Medicine, 141(6), 1442–1447.
Kondo, M., Kato, H., Yoshikawa, T., & Sugino, S. (1986). Treatment of cancer ascites by intraperitoneal administration of a streptococcal preparation OK-432 with fresh human complement—role of complement-derived chemotactic factor to neutrophils. International Journal of Immunopharmacology, 8(7), 715–721.
Lichtenstein, A. (1987). Stimulation of the respiratory burst of murine peritoneal inflammatory neutrophils by conjugation with tumor cells. Cancer Research, 47(9), 2211–2217.
Lichtenstein, A., & Kahle, J. (1985). Anti-tumor effect of inflammatory neutrophils: characteristics of in vivo generation and in vitro tumor cell lysis. International Journal of Cancer, 35(1), 121–127.
Pickaver, A. H., Ratcliffe, N. A., Williams, A. E., & Smith, H. (1972). Cytotoxic effects of peritoneal neutrophils on a syngeneic rat tumour. Nature - New Biology, 235(58), 186–187.
Inoue, T., & Sendo, F. (1983). In vitro induction of cytotoxic polymorphonuclear leukocytes by supernatant from a concanavalin A-stimulated spleen cell culture. Journal of Immunology, 131(5), 2508–2514.
Colombo, M. P., Lombardi, L., Stoppacciaro, A., Melani, C., Parenza, M., Bottazzi, B., et al. (1992). Granulocyte colony-stimulating factor (G-CSF) gene transduction in murine adenocarcinoma drives neutrophil-mediated tumor inhibition in vivo. Neutrophils discriminate between G-CSF-producing and G-CSF-nonproducing tumor cells. Journal of Immunology, 149(1), 113–119.
Musiani, P., Allione, A., Modica, A., Lollini, P. L., Giovarelli, M., Cavallo, F., et al. (1996). Role of neutrophils and lymphocytes in inhibition of a mouse mammary adenocarcinoma engineered to release IL-2, IL-4, IL-7, IL-10, IFN-alpha, IFN-gamma, and TNF-alpha. Laboratory Investigation, 74(1), 146–157.
Colombo, M. P., Ferrari, G., Stoppacciaro, A., Parenza, M., Rodolfo, M., Mavilio, F., et al. (1991). Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. Journal of Experimental Medicine, 173(4), 889–897.
Aeed, P. A., Nakajima, M., & Welch, D. R. (1988). The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. International Journal of Cancer, 42(5), 748–759.
Aeed, P. A., & Welch, D. R. (1988). Sensitivity of locally recurrent rat mammary tumour cell lines to syngeneic polymorphonuclear cell, macrophage and natural killer cell cytolysis. British Journal of Cancer, 58(6), 746–752.
Dallegri, F., Ballestrero, A., Ottonello, L., & Patrone, F. (1989). Defective antibody-dependent tumour cell lysis by neutrophils from cancer patients. Clinical and Experimental Immunology, 77(1), 58–61.
Trellakis, S., Bruderek, K., Dumitru, C. A., Gholaman, H., Gu, X., Bankfalvi, A., et al. (2010). Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International Journal of Cancer, 129(9), 2183–2193.
Tazzyman, S., Barry, S. T., Ashton, S., Wood, P., Blakey, D., Lewis, C. E., et al. (2011). Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. International Journal of Cancer, 129(4), 847–58.
Shang, K., Bai, Y. P., Wang, C., Wang, Z., Gu, H. Y., Du, X., et al. (2012). Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One, 7(12), e51848.
Tazawa, H., Okada, F., Kobayashi, T., Tada, M., Mori, Y., Une, Y., et al. (2003). Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. American Journal of Pathology, 163(6), 2221–2232.
Ishikawa, M., Koga, Y., Hosokawa, M., & Kobayashi, H. (1986). Augmentation of B16 melanoma lung colony formation in C57BL/6 mice having marked granulocytosis. International Journal of Cancer, 37(6), 919–924.
Jung, M. R., Park, Y. K., Jeong, O., Seon, J. W., Ryu, S. Y., Kim, D. Y., et al. (2011). Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. Journal of Surgical Oncology, 104(5), 504–510.
Shimada, H., Takiguchi, N., Kainuma, O., Soda, H., Ikeda, A., Cho, A., et al. (2010). High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer, 13(3), 170–176.
Ubukata, H., Konishi, S., Nagata, H., Kasuga, N., Watanabe, Y., Goto, Y., et al. (2010). Significance of preoperative evaluations of tumor necrosis factor-alpha, the granulocyte/lymphocyte ratio and their correlation with regard to outcome in gastric cancer patients. Digestive Surgery, 27(4), 324–330.
Ding, P. R., An, X., Zhang, R. X., Fang, Y. J., Li, L. R., Chen, G., et al. (2010). Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. International Journal of Colorectal Disease, 25(12), 1427–1433.
Roxburgh, C. S., Wallace, A. M., Guthrie, G. K., Horgan, P. G., & McMillan, D. C. (2010). Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative surgery for colon cancer. Colorectal Disease, 12(10), 987–994.
Chua, W., Charles, K. A., Baracos, V. E., & Clarke, S. J. (2011). Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. British Journal of Cancer, 104(8), 1288–1295.
Tomita, M., Shimizu, T., Ayabe, T., Yonei, A., & Onitsuka, T. (2011). Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Research, 31(9), 2995–2998.
Sharaiha, R. Z., Halazun, K. J., Mirza, F., Port, J. L., Lee, P. C., Neugut, A. I., et al. (2011). Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Annals of Surgical Oncology, 18(12), 3362–9.
Aliustaoglu, M., Bilici, A., Seker, M., Dane, F., Gocun, M., Konya, V., et al. (2010). The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepato-Gastroenterology, 57(99–100), 640–645.
An, X., Ding, P. R., Li, Y. H., Wang, F. H., Shi, Y. X., Wang, Z. Q., et al. (2010). Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers, 15(6), 516–522.
Bhatti, I., Peacock, O., Lloyd, G., Larvin, M., & Hall, R. I. (2010). Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. American Journal of Surgery, 200(2), 197–203.
Tavares-Murta, B. M., Mendonca, M. A., Duarte, N. L., da Silva, J. A., Mutao, T. S., Garcia, C. B., et al. (2010). Systemic leukocyte alterations are associated with invasive uterine cervical cancer. International Journal of Gynecological Cancer, 20(7), 1154–1159.
Cho, H., Hur, H. W., Kim, S. W., Kim, S. H., Kim, J. H., Kim, Y. T., et al. (2009). Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunology, Immunotherapy, 58(1), 15–23.
Thavaramara, T., Phaloprakarn, C., Tangjitgamol, S., & Manusirivithaya, S. (2011). Role of neutrophil to lymphocyte ratio as a prognostic indicator for epithelial ovarian cancer. Journal of the Medical Association of Thailand, 94(7), 871–877.
Yamashita, J., Ogawa, M., & Shirakusa, T. (1995). Free-form neutrophil elastase is an independent marker predicting recurrence in primary breast cancer. Journal of Leukocyte Biology, 57(3), 375–378.
Jensen, H. K., Donskov, F., Marcussen, N., Nordsmark, M., Lundbeck, F., & von der Maase, H. (2009). Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. Journal of Clinical Oncology, 27(28), 4709–4717.
Kuang, D. M., Zhao, Q., Wu, Y., Peng, C., Wang, J., Xu, Z., et al. (2011). Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. Journal of Hepatology, 54(5), 948–955.
Mentzel, T., Brown, L. F., Dvorak, H. F., Kuhnen, C., Stiller, K. J., Katenkamp, D., et al. (2001). The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Archiv, 438(1), 13–22.
Jensen, T. O., Schmidt, H., Moller, H. J., Donskov, F., Hoyer, M., & Sjoegren, P. (2011). Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer, 118(9), 2476–8.
Liu, H., Ubukata, H., Tabuchi, T., Takemura, A., Motohashi, G., Nishimura, M., et al. (2009). It is possible that tumour-infiltrating granulocytes promote tumour progression. Oncology Reports, 22(1), 29–33.
Mantovani, A. (2009). The yin-yang of tumor-associated neutrophils. Cancer Cell, 16(3), 173–174.
Fridlender, Z. G., Sun, J., Kim, S., Kapoor, V., Cheng, G., Ling, L., et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell, 16(3), 183–194.
Mantovani, A., Sica, A., & Locati, M. (2005). Macrophage polarization comes of age. Immunity, 23(4), 344–346.
Jablonska, J., Leschner, S., Westphal, K., Lienenklaus, S., & Weiss, S. (2010). Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. Journal of Clinical Investigation, 120(4), 1151–1164.
Houghton, A. M. (2010). The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle, 9(9), 1732–1737.
Mishalian, I., Bayuh, R., Levy, L., Zolotarov, L., Michaeli, J., & Fridlender, Z. G. (2013). Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunology, Immunotherapy, 62(11), 1745–1756.
Wu, Q. D., Wang, J. H., Condron, C., Bouchier-Hayes, D., & Redmond, H. P. (2001). Human neutrophils facilitate tumor cell transendothelial migration. American Journal of Physiology - Cellular Physiology, 280(4), C814–822.
Wislez, M., Fleury-Feith, J., Rabbe, N., Moreau, J., Cesari, D., Milleron, B., et al. (2001). Tumor-derived granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor prolong the survival of neutrophils infiltrating bronchoalveolar subtype pulmonary adenocarcinoma. American Journal of Pathology, 159(4), 1423–1433.
Balkwill, F., & Mantovani, A. (2001). Inflammation and cancer: back to Virchow? Lancet, 357(9255), 539–545.
Hannelien, V., Karel, G., Jo, V. D., & Sofie, S. (2011). The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochimica et Biophysica Acta, 1825(1), 117–129.
Murdoch, C., & Finn, A. (2000). Chemokine receptors and their role in inflammation and infectious diseases. Blood, 95(10), 3032–3043.
Waugh, D. J., & Wilson, C. (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741.
Eck, M., Schmausser, B., Scheller, K., Brandlein, S., & Muller-Hermelink, H. K. (2003). Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clinical and Experimental Immunology, 134(3), 508–515.
Bieche, I., Chavey, C., Andrieu, C., Busson, M., Vacher, S., Le Corre, L., et al. (2007). CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocrine-Related Cancer, 14(4), 1039–1052.
Cheng, W. L., Wang, C. S., Huang, Y. H., Tsai, M. M., Liang, Y., & Lin, K. H. (2011). Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Annals of Oncology, 22(10), 2267–2276.
Strell, C., Lang, K., Niggemann, B., Zaenker, K. S., & Entschladen, F. (2010). Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Experimental Cell Research, 316(1), 138–148.
di Celle, P. F., Carbone, A., Marchis, D., Zhou, D., Sozzani, S., Zupo, S., et al. (1994). Cytokine gene expression in B-cell chronic lymphocytic leukemia: evidence of constitutive interleukin-8 (IL-8) mRNA expression and secretion of biologically active IL-8 protein. Blood, 84(1), 220–228.
Green, A. R., Green, V. L., White, M. C., & Speirs, V. (1997). Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. International Journal of Cancer, 72(6), 937–941.
Tjiong, M. Y., van der Vange, N., ten Kate, F. J., Tjong, A. H. S. P., ter Schegget, J., Burger, M. P., et al. (1999). Increased IL-6 and IL-8 levels in cervicovaginal secretions of patients with cervical cancer. Gynecologic Oncology, 73(2), 285–291.
Scheibenbogen, C., Mohler, T., Haefele, J., Hunstein, W., & Keilholz, U. (1995). Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Research, 5(3), 179–181.
Haqqani, A. S., Sandhu, J. K., & Birnboim, H. C. (2000). Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia, 2(6), 561–568.
Schaider, H., Oka, M., Bogenrieder, T., Nesbit, M., Satyamoorthy, K., Berking, C., et al. (2003). Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. International Journal of Cancer, 103(3), 335–343.
Yao, C., Lin, Y., Chua, M. S., Ye, C. S., Bi, J., Li, W., et al. (2007). Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. International Journal of Cancer, 121(9), 1949–1957.
Maus, U. A., Waelsch, K., Kuziel, W. A., Delbeck, T., Mack, M., Blackwell, T. S., et al. (2003). Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. Journal of Immunology, 170(6), 3273–3278.
Vlodavsky, I., Fuks, Z., Ishai-Michaeli, R., Bashkin, P., Levi, E., Korner, G., et al. (1991). Extracellular matrix-resident basic fibroblast growth factor: implication for the control of angiogenesis. Journal of Cellular Biochemistry, 45(2), 167–176.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100(1), 57–70.
Lawrence, M. B., & Springer, T. A. (1993). Neutrophils roll on E-selectin. Journal of Immunology, 151(11), 6338–6346.
Woodfin, A., Voisin, M. B., & Nourshargh, S. (2010). Recent developments and complexities in neutrophil transmigration. Current Opinion in Hematology, 17(1), 9–17.
Opdenakker, G., & Van Damme, J. (2004). The countercurrent principle in invasion and metastasis of cancer cells. Recent insights on the roles of chemokines. International Journal of Developmental Biology, 48(5–6), 519–527.
Piccard, H., Muschel, R. J., & Opdenakker, G. (2012). On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Critical Reviews in Oncology/Hematology, 82(3), 296–309.
Pham, C. T. (2006). Neutrophil serine proteases: specific regulators of inflammation. Nature Reviews Immunology, 6(7), 541–550.
Hager, M., Cowland, J. B., & Borregaard, N. (2010). Neutrophil granules in health and disease. Journal of Internal Medicine, 268(1), 25–34.
Belaaouaj, A., McCarthy, R., Baumann, M., Gao, Z., Ley, T. J., Abraham, S. N., et al. (1998). Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nature Medicine, 4(5), 615–618.
Lee, W. L., & Downey, G. P. (2001). Leukocyte elastase: physiological functions and role in acute lung injury. American Journal of Respiratory and Critical Care Medicine, 164(5), 896–904.
Houghton, A. M., Rzymkiewicz, D. M., Ji, H., Gregory, A. D., Egea, E. E., Metz, H. E., et al. (2010). Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine, 16(2), 219–223.
Wada, Y., Yoshida, K., Hihara, J., Konishi, K., Tanabe, K., Ukon, K., et al. (2006). Sivelestat, a specific neutrophil elastase inhibitor, suppresses the growth of gastric carcinoma cells by preventing the release of transforming growth factor-alpha. Cancer Science, 97(10), 1037–1043.
Gong, L., Cumpian, A. M., Caetano, M. S., Ochoa, C. E., De la Garza, M. M., Lapid, D. J., et al. (2013). Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Molecular Cancer, 12(1), 154.
Clavel, C., Polette, M., Doco, M., Binninger, I., & Birembaut, P. (1992). Immunolocalization of matrix metallo-proteinases and their tissue inhibitor in human mammary pathology. Bulletin du Cancer, 79(3), 261–270.
Hojilla, C. V., Wood, G. A., & Khokha, R. (2008). Inflammation and breast cancer: metalloproteinases as common effectors of inflammation and extracellular matrix breakdown in breast cancer. Breast Cancer Research, 10(2), 205.
Pollard, J. W. (2008). Macrophages define the invasive microenvironment in breast cancer. Journal of Leukocyte Biology, 84(3), 623–30.
Nielsen, B. S., Timshel, S., Kjeldsen, L., Sehested, M., Pyke, C., Borregaard, N., et al. (1996). 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. International Journal of Cancer, 65(1), 57–62.
Nozawa, H., Chiu, C., & Hanahan, D. (2006). Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America, 103(33), 12493–12498.
Bausch, D., Pausch, T., Krauss, T., Hopt, U. T., Fernandez-del-Castillo, C., Warshaw, A. L., et al. (2011). Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis, 14(3), 235–243.
Bekes, E. M., Schweighofer, B., Kupriyanova, T. A., Zajac, E., Ardi, V. C., Quigley, J. P., et al. (2011). Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. American Journal of Pathology, 179(3), 1455–1470.
Nakamura, T., Kuwai, T., Kim, J. S., Fan, D., Kim, S. J., & Fidler, I. J. (2007). Stromal metalloproteinase-9 is essential to angiogenesis and progressive growth of orthotopic human pancreatic cancer in parabiont nude mice. Neoplasia, 9(11), 979–986.
Starkey, J. R., Liggitt, H. D., Jones, W., & Hosick, H. L. (1984). Influence of migratory blood cells on the attachment of tumor cells to vascular endothelium. International Journal of Cancer, 34(4), 535–543.
Huh, S. J., Liang, S., Sharma, A., Dong, C., & Robertson, G. P. (2010). Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Research, 70(14), 6071–6082.
Carmeliet, P., & Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature, 407(6801), 249–257.
Folkman, J. (2002). Role of angiogenesis in tumor growth and metastasis. Seminars in Oncology, 29(6 Suppl 16), 15–18.
Cassatella, M. A. (1999). Neutrophil-derived proteins: selling cytokines by the pound. Advances in Immunology, 73, 369–509.
Benelli, R., Albini, A., & Noonan, D. (2003). Neutrophils and angiogenesis: potential initiators of the angiogenic cascade. Chemical Immunology and Allergy, 83, 167–181.
Scapini, P., Morini, M., Tecchio, C., Minghelli, S., Di Carlo, E., Tanghetti, E., et al. (2004). CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. Journal of Immunology, 172(8), 5034–5040.
Van Coillie, E., Van Aelst, I., Wuyts, A., Vercauteren, R., Devos, R., De Wolf-Peeters, C., et al. (2001). Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent principle. American Journal of Pathology, 159(4), 1405–1414.
Coussens, L. M., Tinkle, C. L., Hanahan, D., & Werb, Z. (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell, 103(3), 481–490.
Coussens, L. M., & Werb, Z. (1996). Matrix metalloproteinases and the development of cancer. Chemical Biology, 3(11), 895–904.
Bergers, G., & Benjamin, L. E. (2003). Tumorigenesis and the angiogenic switch. Nature Reviews Cancer, 3(6), 401–410.
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2(3), 161–174.
Opdenakker, G., Van den Steen, P. E., Dubois, B., Nelissen, I., Van Coillie, E., Masure, S., et al. (2001). Gelatinase B functions as regulator and effector in leukocyte biology. Journal of Leukocyte Biology, 69(6), 851–859.
Ardi, V. C., Kupriyanova, T. A., Deryugina, E. I., & Quigley, J. P. (2007). Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 104(51), 20262–20267.
Deryugina, E. I., Zajac, E., Juncker-Jensen, A., Kupriyanova, T. A., Welter, L., & Quigley, J. P. (2014). Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia, 16(10), 771–788.
Morikawa, K., Kamegaya, S., Yamazaki, M., & Mizuno, D. (1985). Hydrogen peroxide as a tumoricidal mediator of murine polymorphonuclear leukocytes induced by a linear beta-1,3-D-glucan and some other immunomodulators. Cancer Research, 45(8), 3482–3486.
Lambeth, J. D. (2004). NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology, 4(3), 181–189.
Babior, B. M., Lambeth, J. D., & Nauseef, W. (2002). The neutrophil NADPH oxidase. Archives of Biochemistry and Biophysics, 397(2), 342–344.
Fialkow, L., Wang, Y., & Downey, G. P. (2007). Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radical Biology and Medicine, 42(2), 153–164.
Evans, T. J., Buttery, L. D., Carpenter, A., Springall, D. R., Polak, J. M., & Cohen, J. (1996). Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proceedings of the National Academy of Sciences of the United States of America, 93(18), 9553–9558.
Wheeler, M. A., Smith, S. D., Garcia-Cardena, G., Nathan, C. F., Weiss, R. M., & Sessa, W. C. (1997). Bacterial infection induces nitric oxide synthase in human neutrophils. Journal of Clinical Investigation, 99(1), 110–116.
Sandhu, J. K., Privora, H. F., Wenckebach, G., & Birnboim, H. C. (2000). Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. American Journal of Pathology, 156(2), 509–518.
Weitzman, S. A., & Gordon, L. I. (1990). Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood, 76(4), 655–663.
Wilkinson, D., Sandhu, J. K., Breneman, J. W., Tucker, J. D., & Birnboim, H. C. (1995). Hprt mutants in a transplantable murine tumour arise more frequently in vivo than in vitro. British Journal of Cancer, 72(5), 1234–1240.
Tamir, S., & Tannenbaum, S. R. (1996). The role of nitric oxide (NO.) in the carcinogenic process. Biochimica et Biophysica Acta, 1288(2), F31–36.
Knaapen, A. M., Gungor, N., Schins, R. P., Borm, P. J., & Van Schooten, F. J. (2006). Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis, 21(4), 225–236.
Bronte, V., & Zanovello, P. (2005). Regulation of immune responses by L-arginine metabolism. Nature Reviews Immunology, 5(8), 641–654.
Peinado, H., Rafii, S., & Lyden, D. (2008). Inflammation joins the “niche”. Cancer Cell, 14(5), 347–349.
Roy, L. D., Ghosh, S., Pathangey, L. B., Tinder, T. L., Gruber, H. E., & Mukherjee, P. (2011). Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer. BMC Cancer, 11, 365.
Kaplan, R. N., Rafii, S., & Lyden, D. (2006). Preparing the “soil”: the premetastatic niche. Cancer Research, 66(23), 11089–11093.
Yang, L., Huang, J., Ren, X., Gorska, A. E., Chytil, A., Aakre, M., et al. (2008). Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1 + CD11b + myeloid cells that promote metastasis. Cancer Cell, 13(1), 23–35.
De Larco, J. E., Wuertz, B. R., & Furcht, L. T. (2004). The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clinical Cancer Research, 10(15), 4895–4900.
Welch, D. R., Schissel, D. J., Howrey, R. P., & Aeed, P. A. (1989). Tumor-elicited polymorphonuclear cells, in contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proceedings of the National Academy of Sciences of the United States of America, 86(15), 5859–5863.
Crissman, J. D., Hatfield, J., Schaldenbrand, M., Sloane, B. F., & Honn, K. V. (1985). Arrest and extravasation of B16 amelanotic melanoma in murine lungs. A light and electron microscopic study. Laboratory Investigation, 53(4), 470–478.
Dong, C., Slattery, M. J., Liang, S., & Peng, H. H. (2005). Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Molecular & Cellular Biomechanics, 2(3), 145–159.
Slattery, M. J., & Dong, C. (2003). Neutrophils influence melanoma adhesion and migration under flow conditions. International Journal of Cancer, 106(5), 713–722.
Aceto, N., Bardia, A., Miyamoto, D. T., Donaldson, M. C., Wittner, B. S., Spencer, J. A., et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell, 158(5), 1110–1122.
Liotta, L. A., Saidel, M. G., & Kleinerman, J. (1976). The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Research, 36(3), 889–894.
Morimoto-Kamata, R., Mizoguchi, S., Ichisugi, T., & Yui, S. (2012). Cathepsin G induces cell aggregation of human breast cancer MCF-7 cells via a 2-step mechanism: catalytic site-independent binding to the cell surface and enzymatic activity-dependent induction of the cell aggregation. Mediators of Inflammation, 2012, 456462.
Yui, S., Tomita, K., Kudo, T., Ando, S., & Yamazaki, M. (2005). Induction of multicellular 3-D spheroids of MCF-7 breast carcinoma cells by neutrophil-derived cathepsin G and elastase. Cancer Science, 96(9), 560–570.
Acuff, H. B., Carter, K. J., Fingleton, B., Gorden, D. L., & Matrisian, L. M. (2006). Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Research, 66(1), 259–266.
Fox, S., Leitch, A. E., Duffin, R., Haslett, C., & Rossi, A. G. (2010). Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. Journal of Innate Immunity, 2(3), 216–227.
Filardy, A. A., Pires, D. R., Nunes, M. P., Takiya, C. M., Freire-de-Lima, C. G., Ribeiro-Gomes, F. L., et al. (2010). Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. Journal of Immunology, 185(4), 2044–2050.
Broug-Holub, E., Toews, G. B., van Iwaarden, J. F., Strieter, R. M., Kunkel, S. L., Paine, R., 3rd, et al. (1997). Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infection and Immunity, 65(4), 1139–1146.
Pahler, J. C., Tazzyman, S., Erez, N., Chen, Y. Y., Murdoch, C., Nozawa, H., et al. (2008). Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia, 10(4), 329–340.
Silva, M. T. (2011). Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. Journal of Leukocyte Biology, 89(5), 675–683.
Allenbach, C., Zufferey, C., Perez, C., Launois, P., Mueller, C., & Tacchini-Cottier, F. (2006). Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major. Journal of Immunology, 176(11), 6656–6664.
Swierczak, A., Cook, A. D., Lenzo, J. C., Restall, C. M., Doherty, J. P., Anderson, R. L., et al. (2014). The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunology Research, 2(8), 765–776.
Denardo, D. G., Brennan, D. J., Rexhepaj, E., Ruffell, B., Shiao, S. L., Madden, S. F., et al. (2011). Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery, 1, 54–67.
Ries, C. H., Cannarile, M. A., Hoves, S., Benz, J., Wartha, K., Runza, V., et al. (2014). Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell, 25(6), 846–859.
Geissmann, F., Manz, M. G., Jung, S., Sieweke, M. H., Merad, M., & Ley, K. (2010). Development of monocytes, macrophages, and dendritic cells. Science, 327(5966), 656–661.
Borregaard, N., & Cowland, J. B. (1997). Granules of the human neutrophilic polymorphonuclear leukocyte. Blood, 89(10), 3503–3521.
Tazzyman, S., Lewis, C. E., & Murdoch, C. (2009). Neutrophils: key mediators of tumour angiogenesis. International Journal of Experimental Pathology, 90(3), 222–231.
Arenberg, D. A., Kunkel, S. L., Polverini, P. J., Glass, M., Burdick, M. D., & Strieter, R. M. (1996). Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. Journal of Clinical Investigation, 97(12), 2792–2802.
Huang, S., Mills, L., Mian, B., Tellez, C., McCarty, M., Yang, X. D., et al. (2002). Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. American Journal of Pathology, 161(1), 125–134.
Walters, I., Austin, C., Austin, R., Bonnert, R., Cage, P., Christie, M., et al. (2008). Evaluation of a series of bicyclic CXCR2 antagonists. Bioorganic and Medicinal Chemistry Letters, 18(2), 798–803.
Varney, M. L., Singh, S., Li, A., Mayer-Ezell, R., Bond, R., & Singh, R. K. (2011). Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Letters, 300(2), 180–188.
Singh, S., Sadanandam, A., Nannuru, K. C., Varney, M. L., Mayer-Ezell, R., Bond, R., et al. (2009). Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clinical Cancer Research, 15(7), 2380–2386.
Ning, Y., Labonte, M. J., Zhang, W., Bohanes, P. O., Gerger, A., Yang, D., et al. (2012). The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Molecular Cancer Therapeutics, 11(6), 1353–1364.
Coffelt, S. B., Kersten, K., Doornebal, C. W., Weiden, J., Vrijland, K., Hau, C. S., et al. (2015). IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature, 522(7556), 345–8.
Gadducci, A., Sergiampietri, C., & Guiggi, I. (2013). Antiangiogenic agents in advanced, persistent or recurrent endometrial cancer: a novel treatment option. Gynecological Endocrinology, 29(9), 811–6.
Shojaei, F., Wu, X., Qu, X., Kowanetz, M., Yu, L., Tan, M., et al. (2009). G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6742–6747.
Phan, V. T., Wu, X., Cheng, J. H., Sheng, R. X., Chung, A. S., Zhuang, G., et al. (2013). Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proceedings of the National Academy of Sciences of the United States of America, 110(15), 6079–6084.
Shojaei, F., & Ferrara, N. (2008). Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Research, 68(14), 5501–5504.
Sanford, M. A., Yan, Y., Canfield, S. E., Hassan, W., Selleck, W. A., Atkinson, G., et al. (2001). Independent contributions of GR-1+ leukocytes and Fas/FasL interactions to induce apoptosis following interleukin-12 gene therapy in a metastatic model of prostate cancer. Human Gene Therapy, 12(12), 1485–1498.
Siders, W. M., Shields, J., Garron, C., Hu, Y., Boutin, P., Shankara, S., et al. (2010). Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leukemia and Lymphoma, 51(7), 1293–1304.
Hernandez-Ilizaliturri, F. J., Jupudy, V., Ostberg, J., Oflazoglu, E., Huberman, A., Repasky, E., et al. (2003). Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clinical Cancer Research, 9(16 Pt 1), 5866–5873.
Thornton, L. M., Andersen, B. L., & Carson, W. E., 3rd. (2008). Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case–control analysis. Cancer Immunology, Immunotherapy, 57(10), 1471–1481.
Fridlender, Z. G., & Albelda, S. M. (2012). Tumor-associated neutrophils: friend or foe? Carcinogenesis, 33(5), 949–955.
Remedi, M. M., Donadio, A. C., & Chiabrando, G. A. (2009). Polymorphonuclear cells stimulate the migration and metastatic potential of rat sarcoma cells. International Journal of Experimental Pathology, 90(1), 44–51.
Acknowledgments
The authors wish to acknowledge funding from the National Health and Medical Research Council (NHMRC) of Australia, research fellowships to JAH (NHMRC) and RLA (National Breast Cancer Foundation), and scholarship support to AS (NHMRC) and KAM (Monash University).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
John A. Hamilton and Robin L. Anderson joint senior authors
Rights and permissions
About this article
Cite this article
Swierczak, A., Mouchemore, K.A., Hamilton, J.A. et al. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev 34, 735–751 (2015). https://doi.org/10.1007/s10555-015-9594-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-015-9594-9