Abstract
Background
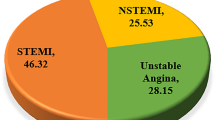
Acute coronary syndrome (ACS) is a serious and life-threatening condition. Anticoagulation during the acute phase of ACS is effective in reducing ischemic events. The most widely used parenteral anticoagulation agent in ACS patients is enoxaparin. Rivaroxaban is a novel oral anticoagulant with potent anti-Xa activity, which might be an attractive alternative drug to enoxaparin. In fact, rivaroxaban was consistently shown to be non-inferior to enoxaparin therapy in terms of reduction of recurrent venous thromboembolism events.
Objective
This prospective, randomized, open-label, active-controlled, multicenter study is designed to compare the safety and efficacy of rivaroxaban versus enoxaparin in patients with ACS, who missed the primary reperfusion therapy window and before selective revascularization.
Methods and Results
Up to 2055 participants receiving background treatment of aspirin plus clopidogrel or ticagrelor will be randomly assigned to either oral rivaroxaban 2.5 mg twice daily or rivaroxaban 5 mg twice daily or subcutaneous enoxaparin 1 mg/kg twice daily until hospital discharge for a maximum of 8 days or 12 h before revascularization therapy. The primary safety endpoint is the International Society on Thrombosis and Hemostasis definition of bleeding events [minor, clinically relevant non-major and major bleeding]. The primary efficacy endpoint is a composite of major adverse cardiac events (MACE), including cardiac death, myocardial infarction, re-revascularization or stroke, and major bleeding events. Secondary endpoints include cardiac-related rehospitalization and all-cause death. Patients will be followed for 12 months after randomization.
Conclusions
The H-REPLACE trial offers an opportunity to assess clinical outcomes of rivaroxaban versus enoxaparin during the acute phase of ACS and may provide an alternative anticoagulation strategy for ACS patients, who missed the primary reperfusion therapy window and before selective revascularization.
Trial Registration
ClinicalTrials.gov; NCT03363035.

Similar content being viewed by others
References
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. AHA/ACC guideline for the Management of Patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–75.
Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776–86.
Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372(9632):31–9.
Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373(9676):1673–80.
Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374(9683):29–38.
Mahaffey KW, Hager R, Wojdyla D, White HD, Armstrong PW, Alexander JH, et al. Meta-analysis of intracranial hemorrhage in acute coronary syndromes: incidence, predictors and clinical outcomes. J Am Heart Assoc. 2015;4(6):e001512.
Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9–19.
Michael GC, Roxana M, Christoph B, Jonathan H, Verheugt Freek W, Peter W, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–34.
Fei S, Mengyue Y, Jingang Y, Haiyan X, Yanyan Z, Wei L, et al. Symptom-onset-to-balloon time, ST-segment resolution and in-hospital mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention in China: from China acute myocardial infarction registry. Am J Cardiol. 2016;118(9):1334–9.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Approval
The H-REPLACE study was approved by the local Institutional Review Board or Ethics Committee at each participating institution.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Xiao, Y., Tang, L., Liu, Q. et al. Rationale and Design of the H-REPLACE Study: Safety and Efficacy of LMWH Versus Rivaroxaban in ChinEse Patients HospitaLized with Acute Coronary SyndromE. Cardiovasc Drugs Ther 36, 69–73 (2022). https://doi.org/10.1007/s10557-020-07076-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07076-9