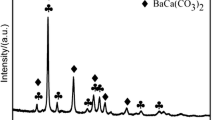
The effects of thermal aging and H2O treatment on the physicochemical properties of BaO/Al2O3 (the NO x storage component in the lean NO x trap systems) were investigated by means of X-ray diffraction (XRD), BET, TEM/EDX and NO2 TPD. Thermal aging at 1000 °C for 10 h converted dispersed BaO/BaCO3 on Al2O3 into low surface area crystalline BaAl2O4. TEM/EDX and XRD analysis showed that H2O treatment at room temperature facilitated a dissolution/reprecipitation process, resulting in the formation of a highly crystalline BaCO3 phase segregated from the Al2O3 support. Crystalline BaCO3 was formed from conversion of both BaAl2O4 and a dispersed BaO/BaCO3 phase, initially present on the Al2O3 support material after calcinations at 1000 and 500 °C, respectively. Such a phase change proceeded rapidly for dispersed BaO/BaCO3/Al2O3 samples calcined at relatively low temperatures with large BaCO3 crystallites observed in XRD within 10 min after contacting the sample with water. Significantly, we also find that the change in barium phase occurs even at room temperature in an ambient atmosphere by contact of the sample with moisture in the air, although the rate is relatively slow. These phenomena imply that special care to prevent the water contact must be taken during catalyst synthesis/storage, and during realistic operation of Pt/BaO/Al2O3 NO x trap catalysts since both processes involve potential exposure of the material to CO2 and liquid and/or vapor H2O. Based on the results, a model that describes the behavior of Ba-containing species upon thermal aging and H2O treatment is proposed.
Similar content being viewed by others
References
W.S. Epling L.E. Campbell A. Yezeretz N.W. Currier J.E. Parks SuffixII (2004) Catal. Rev. Sci. Eng. 46 163 Occurrence Handle10.1081/CR-200031932
S. Matsumoto (1996) Catal. Today 29 43 Occurrence Handle10.1016/0920-5861(95)00259-6 Occurrence Handle1:CAS:528:DyaK28XjtlSlsLw%3D
P. Engström A. Amberntsson M. Skoglundh E. Fridell G. Smedler (1999) Appl. Catal. B 22 L241
L. Lietti P. Forzatti I. Nova E. Tronconi (2001) J. Catal. 204 175 Occurrence Handle10.1006/jcat.2001.3370 Occurrence Handle1:CAS:528:DC%2BD3MXotF2iurw%3D
B.-H. Jang T.-H. Yeon H.-S. Han Y.-K. Park J.-E. Yie (2001) Catal. Lett. 77 21 Occurrence Handle10.1023/A:1012759716973 Occurrence Handle1:CAS:528:DC%2BD3MXptlKqt7s%3D
G.W. Graham H.-W. Jen J.R. Theis R.W. McCabe (2004) Catal. Lett. 93 3 Occurrence Handle1:CAS:528:DC%2BD2cXhsVGqsLo%3D
S. Goňi M.T. Goztaňaga A. Guerrero (2002) J. Mater. Res. 17 1834
H.F.W. Taylor, in: Cement Chemistry, edited by H.F.W. Taylor (Academic Press, London, United Kingdom, 1992), Ch. 10, pp. 333.
F. Prinetto G. Ghiotti I. Nova L. Lietti E. Tronconi P. Forzatti (2001) J. Phys. Chem. B 105 12732 Occurrence Handle10.1021/jp012702w Occurrence Handle1:CAS:528:DC%2BD3MXoslSnt7k%3D
J. Szanyi J.H. Kwak J. Hanson C. Wang T. Szailer C.H.F. Peden (2005) J. Phys. Chem. B 109 7339 Occurrence Handle1:CAS:528:DC%2BD2MXit12ju7Y%3D
J. Szanyi J.H. Kwak D.H. Kim S.H. Burton C.H.F. Peden (2005) J. Phys. Chem. B 109 27 Occurrence Handle1:CAS:528:DC%2BD2cXhtVKitL7P
G. Centi G.E. Arena S. Perathoner (2003) J. Catal. 216 443 Occurrence Handle10.1016/S0021-9517(02)00072-6 Occurrence Handle1:CAS:528:DC%2BD3sXjvVCnu7c%3D
M. Piacentini M. Maciejewski A. Baiker (2005) Appl. Catal. B 59 137
L. Olsson E. Fridell (2002) J. Catal. 210 340 Occurrence Handle10.1006/jcat.2002.3698 Occurrence Handle1:CAS:528:DC%2BD38XmsFWnurY%3D
G.E. Arena A. Bianchini G. Centi F. Vazzana (2001) Top. Catal. 16/17 157 Occurrence Handle10.1023/A:1016607603860 Occurrence Handle1:CAS:528:DC%2BD3MXns1Ggsbk%3D
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, D.H., Chin, YH., Kwak, J.H. et al. Changes in Ba Phases in BaO/Al2O3 upon Thermal Aging and H2O Treatment. Catal Lett 105, 259–268 (2005). https://doi.org/10.1007/s10562-005-8700-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-005-8700-y