Abstract
This work studied the performance of nickel supported on Al2O3 and CexZr1 − xO2/Al2O3 (x = 1.0 and 0.5) catalysts for steam reforming of liquid petroleum gas. Ni/CeO2/Al2O3 and Ni/CeZrO2/Al2O3 exhibited higher stability. In situ XRD, XANES and EXAFS experiments revealed that carbon formation was the main cause for catalyst deactivation.
Graphical Abstract

Similar content being viewed by others
1 Introduction
The growing worldwide energy demand and environmental issues have driven high investments in alternative energy sources. Fuel cells are a promising technology for power generation with high efficiency and low emissions, while respecting environmental limits [1]. Fuel cells require a source of hydrogen to produce electricity.
Currently, the technology for hydrogen production is based on catalytic steam reforming (SR) of hydrocarbons, mostly natural gas [2]. However, the current hydrogen industry does not produce H2 as an energy carrier or as a fuel for energy generation. The installed H2 production and distribution infrastructure is insufficient to support its widespread use in a hydrogen economy. Furthermore, the development of a hydrogen infrastructure is a technical and economic barrier to the use of hydrogen as an energy carrier [3]. Therefore, hydrogen must be produced on site (the so-called distributed hydrogen production) to be economical using a readily available distribution infrastructure of fuels. For stationary power, natural gas is the preferred fuel, where it is available. However, the natural gas infrastructure does not extend to all areas in countries like Brazil. In this particular case, liquefied petroleum gas (LPG), a mixture containing C3H8 and C4H10 in different proportions, has the advantage of a well-established distribution infrastructure, because it is widely used for cooking in large cities as well as in remote areas. Thus, LPG is a fuel with great potential to be used for hydrogen production aiming at generating electricity by fuel cells.
Hydrogen can be produced through the steam reforming (SR) of LPG, which is a highly endothermic reaction (Eq. 1). However, most of the studies found in the literature carried out the SR of propane or butane, separately [4, 5]. There are only few reports in the literature about the production of hydrogen through SR of LPG [6–8]. Furthermore, these studies did not compare the performance of the catalyst for SR of LPG reaction with that one for SR of individual hydrocarbon (propane and butane, separately). For instance, the catalytic oxidation of mixtures of hydrocarbons cannot be predicted directly and linearly from the feed composition, which is attributed to the competitive adsorption of each hydrocarbon that may leads to inhibition effects [9].
Silva et al. [6] carried out a thermodynamic analysis of the SR of LPG [6]. They investigated the effect of the reaction temperature and the steam/carbon ratio on product selectivity and carbon formation. Increasing the reaction temperature and the steam/carbon ratio promoted the production of hydrogen and decreased the formation of carbon.
However, the main issue of this technology is catalyst deactivation mainly due to carbon formation. Park et al. [7] carried out the SR of LPG at 873 K over Ni/Al2O3 and Mn-promoted Ni/Al2O3 catalysts. Significant coke formation was observed over all catalysts but the addition of Mn reduced the amount of carbon deposits. Therefore, the design of a catalyst for SR of LPG that is active and stable under reaction conditions remains a challenge.
Based on the studies related to SR of hydrocarbons, some strategies could be envisaged to improve catalyst stability for SR of LPG. The use of supports with redox properties such as ceria or doped ceria has been extensively reported for methane conversion reactions [10–12]. In this case, the high oxygen mobility of these supports promotes the mechanism of carbon removal, which in turn contributes to the high stability of the catalysts [13]. Different dopants have been reported in the literature but zirconia is one of the most used due to the formation of a ceria-zirconia solid oxide solution that significantly increases the oxygen vacancies of ceria [14].
Therefore, the aim of this work is to study the performance of Ni-based catalysts for SR of LPG. Ceria and zirconia-doped ceria supported on alumina were used as supports in order to improve carbon resistance. In situ X-ray diffraction analyses (XRD) and X-ray absorption spectroscopy (XAS) were performed in order to follow the changes on catalyst structure during the reduction and SR of LPG reaction, which might shed light on the mechanism of catalyst deactivation.
2 Experimental
2.1 Catalysts Preparation
Alumina support was prepared by calcination of bohemite (Puralox–Sasol) at 1073 K in air flow for 6 h. The alumina supported ceria and ceria–zirconia samples were prepared by co-impregnation of the alumina with an aqueous solution of cerium (IV) ammonium nitrate (Sigma–Aldrich) and zirconyl nitrate (Sigma–Aldrich) as precursors of cerium and of zirconium, respectively. The samples were dried at 383 K for 12 h and then calcined in air flow at 1073 K for 5 h. CexZr1 − xO2/Al2O3 samples were prepared with 20 wt.% of ceria or ceria–zirconia oxides and x = 0.5 and 1.0. This ceria or ceria-zirconia content was chosen in order to cover completely the alumina surface [15]. Then, the catalysts were prepared by incipient wetness impregnation of the supports with an aqueous solution of Ni(NO3)2·6H2O (Sigma–Aldrich) to obtain 10 wt.% Ni. The samples were dried at 393 K and calcined under air (50 mL/min) at 673 K for 3 h. The following samples were synthesized: (a) Ni/Al2O3 (Ni/Al); (b) Ni/CeO2/Al2O3 (Ni/Ce/Al); and (c) Ni/Ce0.5Zr0.5O2/Al2O3 (Ni/CeZr/Al).
2.2 X-ray Fluorescence (XRF)
The chemical composition of each sample was determined on a RIGAKU RIX 3100 X-ray spectrometer with a rhodium tube operated at 4 kW. Calcined samples (0.5 g) were analyzed as self-supported wafers. The measurement technique applied was based on a calibration curve obtained using standards.
2.3 BET Surface Area
The BET surface areas of the samples were measured using a Micromeritics ASAP 2020 analyzer by nitrogen adsorption at the boiling temperature of liquid nitrogen.
2.4 Oxygen Storage Capacity (OSC)
Oxygen storage capacity measurements were carried out in a multipurpose unit connected to a quadrupole mass spectrometer (Balzers, Omnistar). The samples (160 mg) were reduced under H2 at 1073 K for 1 h. Then, the samples were cooled to 723 K under He and a 5 % O2/He mixture was passed through the catalyst until the oxygen uptake was finished. The reactor was purged with He and the dead volume was obtained by switching the gas to the 5 % O2/He mixture. Finally the amount of oxygen consumed on the catalysts was calculated taking into account a previous calibration of the mass spectrometer.
2.5 In Situ X-ray Diffraction (XRD)
In situ XRD was carried out at the XPD-10B beam line of the Brazilian Synchrotron Light Laboratory (LNLS). The samples were first crushed, sieved to particle sizes smaller than 20 μm and homogeneously distributed over the support. XRD patterns were then acquired during heating of the samples from room temperature to 1073 K (5 K/min) and hold at this temperature for 30 min, under a H2/He (5 vol.%) mixture (200 mL/min). Then, the sample was cooled to 873 K under He and the reactant mixture containing 60 % H2O, 9 % C3H8, 8 % C4H10 and 23 % Ar was passed through the catalyst during 1h. Scans were carried out from 20° to 55°, using a wavelength of 1.5502 Å.
2.6 In-Situ XAFS
In situ XAS experiments were performed at the D04B-XAFS—1 beamline of the Brazilian Syncrotron Light Source (LNLS, Campinas). Typically, wafers containing pressed mixtures of the sample with BN as a binder were then placed into a sample holder located inside a quartz tubular reactor, which temperature was controlled by a furnace. EXAFS spectra were successively recorded within an energy bracket around the Ni K edge (E0 = 8.333 keV), while the sample underwent the following treatment: (a) reduction under a 5 % H2/He mixture from 298 to 1073 K (10 K/min); (b) temperature hold for 1h at 1073 K; (c) sample cooling down to 298 K, under a 5 % H2/He mixture flow; (d) recording EXAFS spectra at 298 K. Data reducing was made using the ATHENA software package [16]. The background subtracted, normalized XANES spectra were then trimmed at a suitable energy range (c.a. 100 eV above the absorption edge). The Ni containing phase composition during the sample activation (reduction under H2/He flow) was monitored by linear combination of normalized Ni K-edge XANES spectra (LC-XANES) within the 8.3–8.43 keV energy range, using Ni foil and NiO as references for Nio and Ni2+, respectively. For EXAFS data reduction, edge jumps were calibrated referred to the first inflexion point in the edge of a reference of Ni foil placed between the I1 and I2 detection chambers. The k2χ(k) functions were Fourier transformed within a 1.4–2.7 Hanning window, unless stated otherwise in the present work. EXAFS R-space fitting was performed using the FEFF 6 software [17], by considering the amplitudes and phase shifts generated from the scattering paths through the first coordination shell of references: Ni foil and NiO. Data fitting allowed the estimation of the structural parameters N, R and σdw, which are the average number of neighboring scatterers around the absorber atom, the interatomic bond lengths and the Debye–Waller factor, respectively.
2.7 Reaction Conditions
SR of LPG (45 % C3H8, 41 % C4H10 and 14 % Ar) was performed in a quartz reactor at atmospheric pressure. In order to avoid hot spot formation or temperature gradients, catalyst samples (10 mg) were diluted with SiC (90 mg). Prior to the reaction, the catalyst was reduced under H2 at 1073 K for 1 h and then cooled to 873 K under N2. The reaction was carried out at 873 K and a WHSV = 14.31 min−1 over all catalysts. A reactant mixture with H2O/CnH2n + 2 molar ratio of seven and a flow rate of 200 mL/min was used. According to our previous work [6], these reaction conditions favor the formation of carbon and then, they were chosen to investigate the resistance of the catalysts to carbon formation. The SR of propane, butane and methane at 873 K over Ni/Ce/Al was also carried out. In this case, the concentration of each hydrocarbon in the feed corresponded to the sum of both hydrocarbons for SR of LPG. The exit gases were analyzed using a gas chromatograph (Shimadzu) equipped with a thermal conductivity detector and a Carboxen 1010 column (Supelco).
2.8 Thermogravimetric Analysis (TG)
Thermogravimetric analysis of the used catalysts was carried out in a TA Instruments equipment (SDT Q600) in order to determine the amount of carbon formed over the catalyst. Approximately 10 mg of catalyst was heated under air flow from 293 to 1273 K at a heating rate of 20 K/min and the weight change was measured.
3 Results and Discussion
3.1 Catalyst Characterization
Table 1 lists the Ni, CeO2 and ZrO2 content of all samples. The Ni loadings obtained by XRF analysis were close to the nominal value (10 wt.% Ni) for all samples studied. The values of BET surface areas obtained for the supports and catalysts are also shown in Table 1. The addition of ceria or ceria-zirconia did not change significantly the alumina surface area. When Ni was added, the surface area of the support decreased, which is likely due to partial blockage of pores of the supports.
The oxygen uptakes measured for Ni/Ce/Al and Ni/CeZr/Al catalysts are reported in Table 2. The oxygen consumption due to Ni oxidation was discounted from these values. The incorporation of ZrO2 into CeO2 lattice increased the oxygen consumption because of the highest amount of oxygen vacancies of the mixed oxide [18]. The enhancement of oxygen vacancies is due to the high oxygen mobility in the solid solution formed as revealed by XRD data that will be presented next.
3.2 In Situ Characterization Under Reduction and Reaction Conditions
In situ XRD and X-ray absorption spectra on the Ni K-edge (8.333 keV) were carried out in order to investigate the changes in the structure of the metallic phase during reduction under 5 % H2/He flow and upon exposure to SR of LPG reaction.
Figure 1a shows the XRD patterns of the Ni/Al sample during the reduction under a 5 %H2/He mixture from 298 to 1073 K. The diffractogram of the calcined sample (green curve) exhibited the lines typical of γ and θ-alumina phases and NiO cubic phase at 2θ = 43.9°. However, the presence of nickel aluminate phase could not be rule out because their lines are too close to the ones of γ-Al2O3 [19].
a Diffractograms obtained during reduction of Ni/Al under 5 %H2/He mixture at a heating rate of 10 K/min from 298 to 1073 K; b X-ray diffraction patterns of calcined sample (green curve), reduced sample at 1073 K (red curve) and after reduction at 1073 K for 1 h (blue curve); c diffractograms of the sample reduced at 1073 K for 1 h (blue curve) and after SR of LPG for 1 h (black curve). Filled square γ and θ-alumina phases (γ-alumina-PDF#10-0425; θ-alumina-PDF#11–0517); filled circle NiO (PDF#34–0394); filled triangle Nio
Increasing the temperature led to the disappearance of the line attributed to NiO whereas the lines corresponding to Nio at 2θ = 44.5 and 51.7° appeared at around 500 K. The lines assigned to NiO phase were no longer detected at 1073 K. The intensity of the lines related to metallic Ni levels off at 1073 K and remains constant. These changes as a function of reduction temperature are better viewed in Fig. 1b, which showed the diffractograms of the sample when the reduction temperature (1073 K) was achieved (red curve) and after reduction for 1 h at 1073 K (blue curve). The diffractogram remained unchanged after reduction for 1 h, exhibiting the lines typical of metallic Ni and alumina.
After reduction at 1073 K for 1 h, Ni/Al catalyst was cooled to 873 K and exposed to the reaction mixture and the diffractogram obtained after 1 h of time on stream (TOS) was shown in Fig. 1c (black curve). It was not observed significant changes in comparison to the diffractogram of the reduced sample (blue curve). The only difference was the appearance of a broad peak at around 2θ = 26.0°, indicating the presence of carbon deposits [20].
The diffractograms of the Ni/Ce/Al sample during the reduction under a 5 % H2/He mixture from 298 to 1073 K are shown in Fig. 2a. Besides the lines characteristics of alumina and NiO, the diffractogram of the calcined sample exhibited the lines of ceria with cubic structure at 2θ = 28.7°; 33.2° and 47.7°. These lines are slightly shifted due to the wavelength used (1.5502 Å). When the sample was treated under 5 % H2/He mixture, NiO was completely reduced to Nio at around 1073 K (red curve, Fig. 2b). In addition, the intensity of the lines related to metallic Ni increased during reduction (blue curve), suggesting the growth of Ni particle size. It was also noticed the appearance of lines at around 2θ = 33.8 and 41.6° that corresponds to the formation of CeAlO3 (PDF 81-1185). After SR of LPG at 873 K for 1h, the diffractogram remains unchanged, whereas the line corresponding to carbon was not detected (black curve, Fig. 2c).
a Diffractograms obtained during reduction of Ni/Ce/Al under 5 %H2/He mixture at a heating rate of 10 K/min from 298 to 1073 K; b X-ray diffraction patterns of calcined sample (green curve), reduced sample at 1073 K (red curve) and after reduction at 1073 K for 1 h (blue curve); c diffractograms of the sample reduced for 1 h (blue curve) and after SR of LPG for 1 h (black curve). Filled square γ and θ-alumina phases (γ-alumina-PDF#10-0425; θ-alumina-PDF#11–0517); filled diamond CeO2 (PDF#34–0394); filled inverted triangle CeAlO3; filled circle NiO (PDF#34–0394); filled upright triangle Nio
For Ni/CeZr/Al calcined sample, the lines characteristic of CeO2 with cubic structure were shifted to higher 2θ positions (29.4°; 33.9° and 48.8°). This result suggests the formation of a solid solution between the cerium and zirconium oxides [8, 19] (Fig. 3a). The changes in the diffractograms during reduction were quite similar to that of the Ni/Ce/Al sample (Fig. 2a). The formation of Nio begins at 680 K and the reduction is complete at 1073 K. Furthermore, the lines characteristic of ceria were shifted to lower 2θ positions (29.2°; 33.7° and 48.4°) after reduction at 1073 K, which corresponds to the partial reduction of ceria-zirconia solid solution. According to the literature [14], the addition of zirconium to the lattice of ceria increases the number of oxygen vacancies. The highest mobility of oxygen promotes the reducibility of the cerium–zirconium mixed oxide. This result is in agreement with our OSC measurements.
a Diffractograms obtained during reduction of Ni/CeZr/Al under 5 %H2/He mixture at a heating rate of 10 K/min from 298 to 1073 K; b X-ray diffraction patterns of calcined sample (green curve), reduced sample at 1073 K (red curve) and after reduction at 1073 K for 1 h (blue curve); c diffractograms of the sample reduced for 1 h (blue curve) and after SR of LPG for 1 h (black curve). Filled square γ and θ-alumina phases (γ-alumina-PDF#10-0425; θ-alumina-PDF#11–0517); filled diamond CeO2 (PDF#34–0394); filled circle NiO (PDF#34–0394); filled triangle Nio
The diffractogram of Ni/CeZr/Al catalyst after SR of LPG at 873 K for 1 h was very similar to that of the reduced sample (Fig. 3c). In addition, the lines attributed to carbon deposits were not observed.
The crystallite sizes of CeO2 and Nio under different treatments were listed in Table 3. For this calculation, the CeO2 (111) and Nio (200) reflections were used. The most intense line typical of Nio (111) at 2θ = 44.5° was not used because it was not clearly distinguished due to the overlapping with the line characteristic of alumina. The CeO2 crystallite sizes of Ni/Ce/Al and Ni/CeZr/Al catalysts were quite similar (5.0–5.2 nm), regardless of the treatment (calcination, reduction or reaction). The Ni crystallite size of reduced Ni/Al was slightly lower than that of Ni/Ce/Al and Ni/CeZr/Al catalysts. In addition, it was noticed that the Nio crystallite size slightly decreased during the reaction and this change was more significant for ceria-containing catalysts. In order to determine the causes of the changes in the Nio crystallite size during reaction, in situ XAS experiments were carried out and they are presented next.
Figures 4a and 5a shows Ni K-edge XANES spectra of Ni/Al and Ni/Ce/Al catalysts, respectively, recorded during reduction under 5 % H2/He mixture from 298 to 1073 K (green curves) and SR of LPG reaction at 873 K (blue curves). It is noticed that the intensity of the white line decreases as the reduction temperature increased up to 1073 K, which suggests the progressive reduction of NiO to metallic Ni. For both catalysts, the complete reduction of nickel oxide was achieved at around 1073 K. There are no significant changes on XANES spectra during reduction for 1 h. Then, the reaction mixture was flowed through the sample for 1 h. The XANES spectra of Ni/Ce/Al catalyst remained unchanged and similar to the spectra of reduced Ni. However, the XANES spectra underwent changes when Ni/Al catalyst was treated under SR of LPG conditions. The intensity of the first peak slightly increased, indicating that Ni was partially oxidized. These changes can be clearly observed in Fig. 4b.
The evolution of the Ni species during reduction calculated by the linear combination of Ni K-edge XANES spectra of references is shown in Figs. 4b and 5b. The best fit for the linear combination was obtained considering the presence of Nio, NiO and NiAl2O4 references. The calcined samples contain NiO and NiAl2O4 and the distribution of both phases is approximately the same for Ni/Al and Ni/Ce/Al. The reduction of NiO and NiAl2O4 phases and the production of Nio began at 623 K (Ni/Al) and 523 K (Ni/Ce/Al). For both samples, nickel was completely reduced to metallic Ni at 1073 K. When the reaction mixture was admitted into the reaction chamber at 873 K, a very small fraction of metallic Ni (around 5 %) was oxidized by the steam from the feed for Ni/Al catalyst. However, oxidation of metallic Ni was not observed for Ni/Ce/Al catalyst. This result has been reported for the dry reforming of methane over the same catalysts and it is likely due to the preferential oxidation of CeO2 that inhibited the oxidation of metallic Ni particles by the CO2 of the feed [13].
The k2-weighed k and r space EXAFS for the reduced catalysts at 1073 K, along with their respective first coordination shell fits are displayed in Fig. 6. Both samples (Ni/Al and Ni/Ce/Al) reduced at 1073 K were well fitted using Ni fcc, which indicates that Ni is mostly in its metallic state after reduction. Best fittings of Ni K-edge EXAFS spectra recorded at 298 K under H2/He mixture are listed in Table 4. The average coordination number of Ni–Ni of Ni/Ce/Al was slightly smaller than that one of Ni/Al catalyst, whereas the Ni–Ni distance was exactly the same and corresponding to the distance of metallic Ni.
3.3 Steam Reforming of LPG
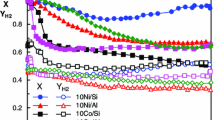
The propane and butane conversion and product distribution as function of TOS obtained for SR of LPG at 873 K over all catalysts is shown in Fig. 7a–e. Ni/Ce/Al catalyst exhibited the highest initial propane conversion. Products distribution was essentially the same for all catalysts (approximately 82 % H2, 6 % CO, 11 % CO2 and 1 % CH4). Ethylene and ethane were not observed for all catalysts. The calculated composition for the thermodynamic equilibrium under this reaction conditions is: 69 % H2, 12 % CO, 8 % CO2 and 10 % CH4 [6]. These results show that the reaction conditions used in the present work are far from the thermodynamic equilibrium.
The formation of H2 and CO indicates that SR of LPG takes place (Eq. 1). However, the higher H2/CO ratio (13.7) than that expected for the SR of LPG reaction (2.3) and the formation of CO2 suggests that the Boudouard reaction (Eq. 2) also occurs under this reaction conditions. It was also observed the formation of small amounts of methane, which could be attributed to the decomposition of hydrocarbons followed by SR of methane as shown by Eqs. (3–7) [22]. These reactions may explain the absence of ethylene or ethane in our work. The occurrence of these reactions has also important implications on catalyst stability as it will be discussed later.
Moon et al. [23] carried out the SR of LPG over Ni-based catalysts derived from Mg-Al hydrotalcite type oxide. The main products formed were H2, CO, CO2 and CH4. The formation of ethane and ethylene has also been reported in the literature due to the decomposition of LPG [23]. Laosiripojana et al. [22] studied the SR of LPG reaction at different temperatures (1023–1173 K) over Ni/Al catalyst, using a H2O/LPG ratio equal to four. They reported that increasing the reaction temperature increases the CO, CO2, CH4 and H2 selectivity and decreases ethylene and ethane selectivity. The authors found values of CO, CO2, CH4, C2H4, C2H6 and H2 at 1023 K, equal to 27, 10, 10, 22, 8 and 23 %, respectively.
In order to investigate the effect of the mixture of hydrocarbons on the performance of the catalysts, the SR of propane or butane over Ni/Ce/Al catalyst was carried out. The propane and butane conversion and product distribution were shown in Fig. 8. For SR of propane (Fig. 8a), the initial propane conversion was around 62 %, which is higher than that one for SR of LPG. Product distribution was quite similar to that one obtained for SR of LPG, with 82 % H2, 4 % CO, 13 % CO2 and 1 % CH4. When butane was used as the feed, the initial butane conversion (49 %) was slightly lower than that one observed for SR of LPG. In this case, the selectivities to CO and CO2 were the same (10 %), the formation of H2 was around 80 % and only trace amounts of methane were detected. These results revealed that the performance of the catalyst is significantly affected in the presence of both hydrocarbons.
Since it is suggested that the mechanism of SR of LPG involves the decomposition of both hydrocarbons and the SR of methane formed, this reaction was also carried out over Ni/Ce/Al catalyst (Fig. 8c). The initial methane conversion was quite low (18 %), and H2, CO2 and CO were the main products formed.
In our work, Ni/Al catalyst significantly deactivated during SR of LPG reaction whereas Ni/Ce/Al and Ni/CeZr/Al catalysts exhibited lower deactivation. Catalyst deactivation during SR of LPG has been reported in the literature. The activity of Ni-based catalysts derived from Mg-Al hydrotalcite type oxide for SR of LPG also decreased during TOS. TEM images and TG analysis showed an important carbon deposition on the catalysts [23]. In order to investigate the main causes of the deactivation of Ni-based catalysts in our work, TG analysis of used catalysts was carried and the results are discussed in the following session.
3.4 TG Analysis of Spent Catalysts
TPO profiles of used catalysts after SR of LPG are shown in Fig. 9. Ni/Al catalyst showed only a peak at 895 K, whereas Ni/Ce/Al and Ni/CeZr/Al exhibited a peak at high temperature region (896 and 910 K, respectively) and a shoulder at 766 and 816 K, respectively.
The total amount of carbon deposited on all catalysts after different reaction conditions, as determined by TG analysis, is listed in Table 5. After SR of LPG at 873 K, the largest amount of carbon was formed on Ni/Ce/Al. However, these catalysts exhibited rather different deactivation behavior, which does not allow any direct comparison concerning the amount of carbon formed between them. In this case, a normalization of the results was performed based on propane conversion and the total amount of carbon deposited was calculated in mgC/gcat/h/mol of converted propane and the results are listed in Table 5. The lowest carbon deposition was observed on Ni/Ce/Al and Ni/CeZr/Al catalysts. Furthermore, Table 5 also lists the amount of carbon deposit in the peak at low temperature region. It is clear that the majority of carbon is oxidized in this region for ceria containing catalysts.
3.5 General Discussion
Catalyst deactivation is one of the main issues for SR of LPG [23]. Catalyst deactivation during SR of hydrocarbons is generally attributed to: (a) the sintering of metallic particles; (b) the oxidation of metallic particles; or (c) the deposition of carbonaceous species. The main causes of deactivation of Ni-based catalysts during SR of LPG are discussed next.
In situ XRD experiments revealed that the crystallite sizes of Nio did not significantly change during 1 h of TOS for all samples (Table 3). In fact, the crystallite size slightly decreased indicating that the deactivation observed cannot be attributed to the sintering of metallic phase.
XANES analyses carried out during SR of LPG showed the appearance of the lines associated with NiO for Ni/Al catalyst, indicating that the metallic Ni particles were partially oxidized by the H2O of the feed during the reaction. However, the degree of oxidation was very low and could not contribute significantly to the catalyst deactivation observed.
In order to investigate the formation of carbon during the reaction, the post-reaction catalysts were characterized by TG analysis. The TPO profile revealed the formation of carbon over all catalysts during SR of LPG.
The two different oxidation regions observed in our TPO profiles can either be due to the presence of two different types of carbon or to a different location of the carbon on the surface of the catalyst. Noronha et al. [12] also observed the presence of two peaks in the TPO profile of Pt/CexZr1 − xO2 catalysts after CO2 reforming of methane. In situ XPS analysis of the used catalysts showed that the different peaks in the TPO were not due to different forms of carbon, but rather to different locations on the catalyst surface. They observed that the Pt/Al2O3 catalyst exhibited only one peak in the high-temperature region whereas the Pt/CexZr1 − xO2 showed the peak at low temperature more prominent. This high temperature peak was assigned to the oxidation of carbon on the support, while the low temperature peak was due to the oxidation of carbon deposits close to the metallic particle that is easily oxidized. This was attributed to the higher oxygen mobility of ceria and ceria-zirconia mixed oxide, which promoted the oxidation of carbon deposits in the metal-support interface. For alumina supported catalyst, in the absence of this oxygen transfer mechanism, carbon is not continuously removed during the reaction and then it accumulates on the surface, resulting in catalyst deactivation. These results are in agreement with the higher stability of Ni/Ce/Al and Ni/CeZr/Al catalysts for SR of LPG reaction.
Laosiripojana et al. [22] also suggested that ceria support decreased the formation of carbon during SR of LPG. They investigated the steam reforming of LPG at different temperatures (1023–1173 K) over Ni/GdCeO2, Ni/Al2O3, Rh/Al2O3 and Rh/GdCeO2. After reaction at 1173 K for 10 h, large amounts of carbon were formed over all catalysts. However, GdCeO2 supported catalysts exhibited lower degree of carbon formation. This higher resistance to carbon deposition was attributed to the oxygen mobility of ceria. According to the authors, lattice oxygen reacts with carbon species formed on the surface, inhibiting carbon accumulation.
The mechanism of SR of higher hydrocarbons involves the adsorption of the hydrocarbon on the surface of Ni particle followed by C–C bond breaking, [24, 25]. Density functional theory (DFT) demonstrated that cleavage of C–C bonds for propane and butane occurs via α,β-bound CH3C*–C*H and CH3C*–C*CH3 transition states respectively, which requires two sites on the catalyst surface [25]. Then, the following reactions take place:
C–C bond cleavage is the sole kinetically relevant step and H–H and C–H bond dissociation steps are considered to be quasi-equilibrated. Further reactions leads to the formation of CHx species on the metal surface. These CHx species formed may reacts with adsorbed H atoms producing CH4 or it may be further dehydrogenated to H and C. Therefore, if the mechanism of SR of LPG involves the decomposition of both hydrocarbons to methane, a large formation of CH4 should be observed because the activity of Ni/Ce/Al catalyst to SR of methane was low under this reaction conditions. However, the amount of methane formed during SR of LPG was too low. These results suggest that SR of methane does not occur in a significant extent in our SR of LPG reaction conditions, as expected by the literature [25]. Therefore, catalyst activity in LPG reforming is not dependent on its activity in methane reforming. Once the CHx species are formed they rapidly decompose to H and C, which may reacts with water producing H2 and CO.
For ceria-based catalysts, this carbon may also react with the oxygen from the support, producing CO and an oxygen vacancy. The oxygen vacancy formed is replenished by water or CO2 produced in the reaction, keeping the redox mechanism active.
The reaction pathways for SR of LPG over Ni-supported on reducible oxides are shown in Scheme 1. Then, carbon deposition will depend on the balance between the rate of CHx species formation and the rate of carbon removal. In the absence of ceria support, carbon formed may accumulate on the surface or may dissolve in the nickel lattice, which leads to the growth of carbon filaments.
The differences in catalyst activity under the reaction mixture and the individual hydrocarbons are likely due to the dependence of the rate equation for C–C bond cleavage reactions. The alkane hydrogenolysis turnover rate is proportional to alkane pressure and depends inversely on H2 pressure [26]. Therefore, the lower concentration of propane in the reaction mixture should lead to lower propane conversion than for the SR of propane. Furthermore, the alkane hydrogenolysis turnover rates increase with chain length and then, much higher turnover rates are expected for C–C cleavage in butane than propane. Then, the butane conversion is less affected by the reaction mixture.
4 Conclusions
The SR of LPG was performed on Ni supported on Al2O3 and CexZr1 − xO2/Al2O3 (x = 1.0 and 0.5) catalysts. Ni/Al catalyst significantly deactivated during reaction whereas conversion only slightly decreased for Ni/Ce/Al and Ni/CeZr/Al catalysts. In situ techniques (XRD and XANES) allowed to monitor the changes in catalyst structure under reduction and SR of LPG, which has not been previously reported in the literature. This approach shed light on the mechanism of catalyst deactivation during the SR of LPG reaction. In situ XRD experiments showed that the crystallite sizes of Nio did not significantly change during reaction for all samples, which indicates that the sintering of metallic phase is not responsible for the deactivation observed. XANES spectra revealed that the metallic Ni particles were partially oxidized by the H2O of the feed during the reaction over Ni/Al catalyst. However, the extent of oxidation was too limited and could not contribute significantly for the loss of activity. Catalyst deactivation during SR of LPG was mainly due to carbon deposition, as shown by TG analysis. The reaction pathways for SR of LPG over Ni-supported catalysts involve the adsorption of the hydrocarbon on the surface of Ni particle followed by C–C bond breaking, producing CHx species. These CHx species may be further dehydrogenated to H and C. For ceria-based catalysts, this carbon may react with the oxygen from the support, producing CO and inhibiting carbon deposition. The oxygen vacancy generated is replenished by water from the feed, keeping the redox mechanism active. For Ni/Al catalyst that does not exhibit oxygen mobility as ceria materials, carbon will accumulate on the surface of Ni particles or may dissolve in the nickel lattice, leading to the growth of carbon filaments.
References
Gur TM (2016) Prog Energ Combust Sci 54:1–64
Rostrup-Nielsen JR, Sehested J, Norskov JK (2002) Adv Catal 47:65–139
Yang C, Ogden J (2007) Int J Hydrogen Energy 32:268–286
Lim S, Lee H, Moon D, Kim J, Park N, Shin J, Kim Y (2009) Chem Eng J 152:220–226
Ferrandom M, Kropf AJ, Krause T (2010) Appl Catal A Gen 379:121–128
Silva PP, Ferreira RA, Nunes JF, Sousa JA, Romanielo LL, Noronha FB, Hori CE (2015) Braz J Chem Eng 32:647–662
Park JH, Lee D, Lee HC, Park ED (2010) Korean J Chem Eng 27:1132–1138
Gokaliler F, Çaglayan BS, Olsan ZI, Aksoylu AE (2008) Int J Hydrogen Energy 33:1383–1391
Spivey J (1987) Ind Eng Chem Res 26:2165–2180
Passos FB, de Oliveira ER, Mattos LV Noronha F (2005) Catal Today 101:23–30
Ruiz JAC, Passos FB, Bueno JMC, Souza-Aguiar EF, Mattos LV, Noronha FB (2008) Appl Catal 334:259–267
Noronha FB, Fendley EC, Soares RR, Alvarez, WE, Resasco DE (2001) Chem Eng J 82:21–31
Faria EC, Neto RCR, Colman RC, Noronha FB (2014) Catal Today 228:138–144
di Monte R, Kaspar JJ (2005) Mater Chem 15:633–648
Silva FA, Martinez DS, Ruiz JAC, Mattos LV, Hori CE, Noronha FB (2008) Appl Catal A Gen 335:145–152
Newville MJ (2001) Synchrotron Radiat 8:96–100
Rehr JJ, Zabinsky SI, Albers RC (1992) Phys Rev Lett 69:3397
Hori CE, Permana H, Simon NGKY, Brenner A, More K, Rahmoeller KM, Belton D (1998) Appl Catal B Env 16:105–117
Roh HS, Jun KW, Park SE (2003) Appl Catal A 251:275
Zhang H, Chu W, Zou C, Huang Z, Ye Z, Zhu L (2011) Catal Lett 141:438–444
Trovarelli A, Zamar F, Llorca J, Leitenburg C, Dolcetti G, Kiss JT (1997) J Catal 169: 490
Laosiripojana N, Sutthisripok W, Charojrochkul S, Assabumrungrat S (2011) Fuel 90:136–141
Moon DJ (2008) Catal Survey Asia 12:188–202
Rostrup-Nielsen JR, Rostrup-Nielsen T (2002) CatTech 6:150–159
Hibbits DD, Flaherty DW, Iglesia E (2016) J Phys Chem C 120:8125–8138
Flaherty DW, Hibbits DD, Iglesia E (2014) J Am Chem Soc 136:9664–9676
Acknowledgments
The authors acknowledge the scholarship and financial support received from CNPq and FAPERJ. The group also thanks LNLS for the assigned beamtime at XAFS-1 and XPD and for the valuable support to perform the XAS and diffraction studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faria, E.C., Rabelo-Neto, R.C., Colman, R.C. et al. Steam Reforming of LPG over Ni/Al2O3 and Ni/CexZr1 − xO2/Al2O3 Catalysts. Catal Lett 146, 2229–2241 (2016). https://doi.org/10.1007/s10562-016-1833-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1833-3