Abstract
This work reports simple, efficient, selective protocol for the oxidation of sulfide compounds. Various sulfides were selectively and completely converted into their corresponding sulfoxides and sulfones using H2O2 as an oxidant in presence of catalytic amount of caprylic acid. The reaction proceeds at room temperature to give sulfoxide and by increasing the reaction temperature to 50 °C, this system provides selective formation of sulfone with high conversion and excellent yields. Green, convenient, easy work-up, chemoselectivity, broad substrate scope and regeneration of catalyst are the important highlights of this protocol.
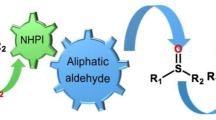
Graphical Abstract

Using H2O2 as an oxidant in presence of catalytic amount of caprylic acid various sulfides were selectively and completely converted into their corresponding sulfoxides and sulfones.


Similar content being viewed by others
References
Frenanez I, Khiar N (2003) Chem Rev 103:3651
Munoza M, Romanellia G, Bottob IL, Cabelloa CI, Lamonierc C (2010) Appl Catal B 100:254
Ghaemi A, Rayati S, Zakavi S, Safari N (2009) Appl Catal A 353:154
Spencer CM, Faulds D (2000) Drugs 103:321
Romanowski G (2013) J Mol Catal A Chem 368:137
Mba M, Prins LJ, Zonta C, Cametti M, Valkonen A, Rissanen K, licini G (2010) Dalton Trans 39:7384
Khurana JM, Nand B (2010) Can J Chem 88:906
Alcon MJ, Corma A, Iglesias M, Sanchez F (2002) J Mol Catal A Chem 178:253
Das R, Chakarborty D (2010) Tetrahedron Lett 51:6255
Mandal M, Chakarborty D (2015) RSC Adv 5:12111
Chen TH, Yuan ZB, Carver A, Zhang R (2014) Appl Catal A 478:275
Bagherzadeh M, Latifi R, Tahsini L, Amini M (2008) Catal Commun 10:196
Matteucci M, Bhalay G, Bradley M (2003) Org Lett 5:235
Gao J, Guo H, Liu S, Wang M (2007) Tetrahedron Lett 48:8453
Raju BR, Sarkar S, Reddy U C, Saikia AK (2009) J Mol Catal A Chem 308:169
Jayaseeli AMI, Rajagopal S (2009) J Mol Catal A Chem 309:103
Gunaratne HQN, McKervey MA, Feutren S, Finlay J, Boyd J (1998) Tetrahedron Lett 39:5655
Mello R, Olmos A, Aragonés AA, Rodríguez AD, Núñez MEG, Asensio G (2010) Eur J Org Chem 32:6200
Karmee SK, Greiner L, Kraynov A, Muller TE, Niemeijer B, Leitner W (2010) Chem Commun 46:6705
Rostami A, Akradi J (2010) Tetrahedron Lett 51:3501
Jafarpour M, Ghahramaninezhad M, Rezaeifard A (2014) New J Chem 38:2917
Golchoubian H, Hosseinpoor F (2007) Molecules 12:304
Gayakwad EM, Patil VV, Shankarling GS (2016) New J Chem 40:223:24.
Tumula VR, Bondwal S, Bisht P, Pendem C, Kumar J (2012) React Kinet Mech Cat 107:449
Dai DY, Wang L, Chen Q, He MY (2014) J Chem Res 38:183
Secci F, Arca M, Frongia A, Piras PP (2014) New J Chem 38:3622
Bahrami K (2006) Tetrahedron Lett 47:2009
Varma RS, Saini RK, Meshram HM (1997) Tetrahedron Lett 38:6525
Rostami A, Tahmasbi B, Abedi F, Shokri Z (2013) J Mol Catal A Chem 378:200
Islam SM, Roy AS, Mondal P, Tuhina K, Mobarak M, Mondal J (2012) Tetrahedron Lett 53:127
Jereb M (2012) Green Chem 14:3047
Wagh RB, Gund SH, Nagarkar JM (2016) J Chem Sci 128:1321
Malkov AV, Derrien N, Barlog M, Kocovsky P (2014) Chem Eur J 20:4542
Acknowledgements
The author (RBW) is greatly thankful to the UGC (University Grant Commission, India) for providing the junior research fellowship (JRF).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wagh, R.B., Nagarkar, J.M. A Simple Metal Free Oxidation of Sulfide Compounds. Catal Lett 147, 181–187 (2017). https://doi.org/10.1007/s10562-016-1932-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1932-1