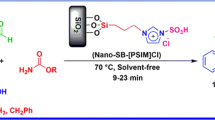
Abstract
To fabricate SiO2/PDA–SO3H nanocatalyst, a suitable method is designed for the loading of sulfonic acid groups on the surface of polydopamine (PDA)-encapsulated SiO2 nanoparticles. To bridge the gap between heterogeneous and homogeneous catalysis, surface functionalization of silica gel is an elegant procedure. The morphology, structure, and physicochemical features were specified using different analytical techniques including field emission scanning electron microscopy (FESEM), Fourier transformed infrared spectroscopy (FT-IR), high resolution-transmission electron microscopy (HR-TEM), energy dispersive X-ray spectroscopy (EDS), wavelength-dispersive X-ray spectroscopy (WDX), X-ray photoelectron spectroscopy (XPS), and back titration. The SiO2/PDA–SO3H nanoparticles are efficient nanocatalysts for the acetylation of many alcohols, phenols, and amines with acetic anhydride under solvent-free conditions in good to excellent yields. Moreover, the reuse and recovery of the catalyst was shown seven times without detectible loss in activity.
Graphical Abstract











Similar content being viewed by others
References
Davies PW (2009) Annu Rep R Soc Chem Sect B Org Chem 105:93–112
Koukabi N, Kolvari E, Zolfigol MA, Khazaei A, Shaghasemi BS, Fasahati B (2012) Adv Synth Catal 354:2001–2008
Deng J, Mo L-P, Zhao F-Y, Hou L-L, Yang L, Zhang Z-H (2011) Green Chem 13:2576–2584
Green TW, Wuts PCM (1999) Protective groups in organic synthesis, 3rd edn. Wiley, New York
Otera J (2003) Esterification: methods, reactions and applications, 1st edn. Wiley, New York
Steglich W, Hofle G (1969) Angew Chem Int Ed 8:981
Vedejs E, Diver TS (1993) J Am Chem Soc 115:3358
Scriven EFV (1983) Chem Soc Rev 12:129
Tomohumi S, Kousaburo O, Takashi O (1991) Synthesis 12:1141
Orita A, Tanahashi C, Kakuda A, Otera J (2000) Angew Chem Int Ed 39:2877
Alleti R, Perambuduru M, Samanha S, Reddy VP (2005) J Mol Catal A Chem 226:57
Karimi B, Maleki J (2003) J Org Chem 68:4951
Ghaffari Khaligh N (2012) J Mol Catal A Chem 363–364:90
Rajabi F (2009) Tetrahedron Lett 50:395
Osiglio L, Sathicq AG, Romanelli GP, Blanco MN (2012) J Mol Catal A Chem 359:97–103
López I, Bravo JL, Caraballo M, Barneto JL, Silvero G (2011) Tetrahedron Lett 52:3339
Tamaddon F, Amrollahi MA, Sharafat L (2005) Tetrahedron Lett 46:7841
Gupta R, Kumar V, Gupta M, Paul S, Gupta R (2008) Indian J Chem Sec B 47:1739
Kumar P, Pandey RK, Bodas MS, Dagade SP, Dongare MK, Ramaswamy AV (2002) J Mol Catal A Chem 181:207
Kooti M, Afshar M (2012) Mat Res Bull 47:3473
Khakiani AB, Pourshamsian K, Veisi H (2015) Appl Organomet Chem 29:259
Astruc D, Lu F, Aranzaes JR (2005) Angew Chem Int Ed 44:7852
Veisi H, Mohammadi P, Gholami J (2014) Appl Organomet Chem 28:868
Sheykhan M, Ma’mani L, Ebrahimi A, Heydari A (2011) J Mol Catal A Chem 335:253
Kiasat AR, Nazari S (2012) J Mol Catal A Chem 365:80
Panella B, Vargas A, Baiker A (2009) J Catal 261:88–93
Chouhan G, Wang D, Alper H (2007) Chem Commun 4809:4809
Veisi H, Taheri S, Hemmati S (2016) Green Chem 18:6337
Zhang M, Liu P, Liu YH, Shang ZR, Hu HX, Zhang ZH (2016) RSC Adv 6:106160–106170
Taheri S, Veisi H, Hekmati M (2017) New J Chem (2017) 41:5075–5081
Farahi M, Karami B, Keshavarz R, Khosravian F (2017) RSC Adv 7:46644–46650
Zhao XN, Hu HC, Zhang FJ, Zhang ZH (2014) Appl Catal A Gen 482:258–265
Veisi H (2010) Tetrahedron Lett 51:2109
Veisi H, Sedrpoushan A, Faraji AR, Heidari M, Hemmti S, Fatahi B (2015) RSC Adv 5:68523
Rao ZM, Wu TH, Peng SY (1995) Acta Phys Chim Sin 11:395
Lin Y, Li L, Hu L, Liu K, Xu Y (2014) Sens Actuators B 202:527
Sahu S, Sinha N, Bhutia SK, Majhi M, Mohapatra S (2014) J Mater Chem B 2:3799–3808
Mo X, Lopez DE, Suwannakarn K, Liu Y, Lotero E, Goodwin JG, Lu CQ (2008) J Catal 254:332
Veisi H, Azadbakht R, Saeidifar F, Abdi MR (2017) Catal Lett 147:976
Bonyasi F, Hekmati M, Veisi H (2017) J Colloid Interface Sci 496:177
Farzad E, Veisi H (2018) J Ind Eng Chem 60:114–124
Shahriary M, Veisi H, Hekmati M, Hemmati S (2018) Mat Sci Eng C 90:57–66
Veisi H, Sedrpoushan A, Hemmati S (2015) Apply Organomet Chem 29:825
Pirhayati M, Veisi H, Kakanejadifard A (2016) RSC Adv 6:27252
Veisi H, Azizi S, Mohamadi P (2018) J Clean Prod 170:1536–1543
Heidari F, Hekmati M, Veisi H (2017) J Colloid Interface Sci 501:175–184
Veisi H, Mirshokraie SA, Ahmadian H (2018) Int J Biol Macromol 108:419–425
Lebaschi S, Hekmati M, Veisi H (2017) J Colloid Interface Sci 485:223
Veisi H, Pirhayati M, Kakanejadifard A, Mohammadi P, Abdi MR, Gholami J, Hemmati S (2018) ChemSelect 3:1820–1826
Veisi H, Rashtiani A, Barjasteh V (2016) Appl Organometal Chem 30:231
Veisi H, Najafi S, Hemmati S (2018) Int J Biol Macromol 113:186–194
Maleki B, Azarifar D, Ghorbani-Vaghei R, Veisi H, Hojati SF, Gholizadeh M, Salehabadi H, Moghadam MK (2009) Monatsh Chem 140:1485
Veisi H, Maleki B, Hamelian M, Ashraphi SS (2015) RSC Adv 5:6365
Veisi H, Ghorbani-Vaghei R, Eskandari H, Hemmati S, Rezaei A. Hajinazari S, Heidari Far MR, Entezari A (2011) Phosphorus Sulfur Silicon 186:213
Osiglio L, Romanelli G, Blanco M (2010) J Mol Catal A Chem 316:52
Niknam K, Saberi D (2009) Appl Catal A Gen 366:220
Hajipour AR, Karimi H (2014) Chin J Catal 35:1982–1989
Bhosale MA, Ummineni D, Sasaki T, Nishio-Hamane D, Bhanage BM (2015) J Mol Catal A Chem 404–405:8
Albadi J, Alihosseinzadeh A, Mardani M (2015) Chinese J Catal 36:308–313
Acknowledgements
We are thankful to Payame Noor University, Tehran, Iran, for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veisi, H., Vafajoo, S., Bahrami, K. et al. Preparation of Polydopamine Sulfamic Acid-Functionalized Silica Gel as Heterogeneous and Recyclable Nanocatalyst for Acetylation of Alcohols and Amines Under Solvent-Free Conditions. Catal Lett 148, 2734–2745 (2018). https://doi.org/10.1007/s10562-018-2486-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2486-1