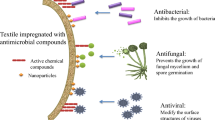
Abstract
Functionalized textiles have been increasingly used for enhancing antimicrobial or antiviral (antipathogenic) action. Those pathogens can cause recurring diseases by direct or indirect transmission. Particularly, airborne microorganisms may cause respiratory diseases or skin infections like allergies and acne and the use of inorganic agents such as metal and metal oxides has proven effective in antipathogen applications. This review is a tutorial on how to obtain functional fabric with processes easily applied for industrial scale. Also, this paper summarizes relevant textiles and respective incorporated inorganic agents, including their antipathogenic mechanism of action. In addition, the processing methods and functional finishing, on a laboratory and industrial scale, to obtain a functional textile are shown. Characterization techniques, including antipathogenic activity and durability, mechanical properties, safety, and environmental issues, are presented. Challenges and perspectives on the broader use of antipathogenic fabrics are discussed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several pathogens such as bacteria, fungi, and viruses are present in everyday life and can cause respiratory problems, skin infections, intestinal complications, and virus diseases. Functionalized textiles can be applied in various areas, mainly in health care, industry (packaging, water disinfection, air filtration, etc.), households, medical devices, clothing and hygiene to prevent the above-mentioned illnesses (Gao and Cranston 2008; Simončič and Tomšič 2017; Ibrahim et al. 2019, 2021; Gulati et al. 2021). Antipathogenic fabrics have broad applicability and can help prevent or solve common issues such as odor releasing or even rare events such as pandemics.
Fabrics from natural or synthetic sources can be used. In the past, synthetic fabrics have dominated the market, but they have been losing ground to cellulose-based fibers (natural from plants and modified). The hydroxyl groups in cellulose give stiffness and strength to the fiber, and they are comfortable and biodegradable. For this reason, they are applied in several areas, which led to a high demand (Uddin 2015; Emam 2019; Felgueiras et al. 2021).
These antipathogenic fabrics can be obtained from the fixation of antipathogenic agents, which can be inorganic or organic and can also be classified as natural or synthetic. The inorganic agents have been emerging due to their antipathogenic potential. And also because they are compatible and present durability in the antipathogenic effect (Raghunath and Perumal 2017).
Various inorganic agents can be used against pathogens, such as silver, copper, titania, or iron oxides (Gao and Cranston 2008; Simončič and Tomšič 2017; Andra et al. 2021). In addition, incorporating antipathogenic agents into textiles or non-woven materials can provide specific or enhanced properties, such as UV-protection, self-cleaning, conductive and others (Gao and Cranston 2008; Raghunath and Perumal 2017; Ibrahim et al. 2017a; Ali et al. 2018, 2021b; Gulati et al. 2021).
In this review, we present and discuss the processing of antipathogenic fabrics functionalized, detailing different fabrics and different agents, manufacturing and finishing technologies for antipathogenic fabrics applicable on an industrial scale, their activity evaluation, their safety, and associated environmental issues.
Textile fibers
Fabrics are formed by fibers, which can be classified on their origin (Fig. 1), as natural fibers, which can be found in plants and animals; and synthetic, obtained by a chemical modification of natural polymers or from polymer synthesis. In general, natural fibers have a short length (stapled fibers), and manufactured fibers are continuous filaments and can be used as a staple (Lord 2003). Both natural and synthetic fibers can favor the growth of pathogens. Synthetic fibers provide a source of nutrition, and the natural fibers contain water, nutrients, and oxygen due to their origin and production (Andra et al. 2021).
The production techniques vary according to the type of fiber and desired product (woven, knit, or non-woven) (Wang et al. 2020a, b), Fig. 2. Woven, knit, and non-woven fabrics are structures that depend on how they are constructed and provide different properties. Woven fabrics have fibers intertwined at right angles (90° angles); a checkered pattern is created by interlacing horizontal fibers over and under vertical fibers. A knit is made by pulling loops of thread, creating a more stretchy sheet than other structures. Non-woven fabrics have the fibers bonded to form a fabric, involving mechanical, chemical, or thermal processes; they are less expensive to produce and have poor "memory" and laundering durability (Songer 2020).

Adapted from Wang et al. (2020b)
Fabric structures.
Natural fibers
Natural fibers can be obtained from plants, such as cotton, flax, hemp, and others; in this case, they are cellulose-based. Alternatively, animal fibers, such as wool, cashmere, silk, and others, are also employed as a raw material; i.e. they can be protein-based. One of the most used fibers is cotton due to its suitable properties such as high strength, long durability, temperature resistance, softness, and breathability. Another natural fiber that also stands out in the textile industry is wool, usually from sheep, which is an excellent thermal insulator. In addition, wool absorbs a large amount of water, has higher temperature resistance than cotton or some synthetic fibers, has a lower rate of flame spread, and is considered hypoallergenic (T for Textile 2021).
Manufactured fibers
Manufactured fibers are obtained from modifying natural polymers such as acetate and viscose or from chemically synthesized polymers such as polyester, polyamide, polyethylene, acrylics, and elastane. In either case, the fibers can be tailored to offer required properties such as strength, durability, and breathability, among others. Those manufactured fibers can present fire resistance, stiffness, high softening point, and melting ability for spinning (T for Textile 2021).
Antipathogenic agents
In a functional textile, the antipathogenic properties are associated with antipathogenic agents, which generally have an inorganic nature. These agents are compounds capable of preventing the growth of microorganisms. Several inorganic agents are reported in the literature. The most studied and explored is silver (Durán et al. 2007; Falletta et al. 2008; Tomšič et al. 2009; Zhang et al. 2009; Kelly and Johnston 2011; Firoz Babu et al. 2012; Balagna et al. 2020; El-Naggar et al. 2020; Tremiliosi et al. 2020), but other particles or ions also have antipathogenic effects, such as copper (Borkow et al. 2010; El-Nahhal et al. 2012; Sójka-Ledakowicz et al. 2016; Xu et al. 2018; Sharma et al. 2019; Vasantharaj et al. 2019), titanium dioxide (Li et al. 2006; Pant et al. 2011; Galkina et al. 2014; Karimi et al. 2014; Prorokova et al. 2018), zinc oxide (El-Nahhal et al. 2017, 2020; Fiedot-Toboła et al. 2018), and iron oxides (Harifi and Montazer 2013; Rastgoo et al. 2016; Qin et al. 2019), as presented in Table 1 and Fig. 3.
Ionic and metallic silver
Silver presents excellent potential as an antipathogenic agent for textile products, both in metallic and ionic forms. Silver is toxic to several microorganisms, but it is non-toxic in low concentrations for humans (Sondi and Salopek-Sondi 2004; Morones et al. 2005; Lara et al. 2010a, b, 2011; Deshmukh et al. 2019). Either in metal or ion form, silver interacts in several molecular processes with the microorganisms and can decrease their growth and infection power. In the case of pathogens, silver can act by destroying the membrane or binding the proteins that cover the pathogens (Deshmukh et al. 2019).
Balagna et al. (2020) coated FFP3 masks with silver nanocluster/silica-based composite as a virucidal agent against SARS-CoV-2. They used radiofrequency in silver nanoclusters and silica-based spraying on the mask surface. In one of the tests, the coating completely removed the cytopathic effect, which can extend the life of the personal protective equipment used.
Ag-based material was incorporated into polycotton fabrics, and the inhibition power of SARS-CoV-2 was evaluated by Tremiliosi et al. (2020). In this case, a 67% polyester and 33% cotton fabric was impregnated with Ag nanoparticles by immersion in two different colloidal solutions (AgNP colloidal solution, CS, and an AgNP colloidal solution stabilized with organic polymers, OP). The experiment reported 99% inhibition of the virus in the tissue with AgOP in 2 min, only 16% inhibition in the untreated tissue, and 86% inhibition in the tissue with AgCS. It was demonstrated that the AgNPs impregnated in the tissue interfere with replication and prevent contamination.
Ali et al. developed nanocomposites containing silver nanoparticles. In this work the main property studied was the detection of Fe3+ ions, but it was also applied in cotton fabrics for antimicrobial evaluation (E. coli, S. aureus, Influenza A virus H3N2, Feline calicivirus FCV and Candida albicans), obtaining good and promising results in all properties tested, creating a multifunctional composite (Ali et al. 2021a).
Another evaluative study was performed by El-Naggar et al. (2020), in which silver nanoparticles (AgNPs) were incorporated into cotton using the pad-dry-cure technique. Besides evaluating the antipathogenic effect, the work synthesized AgNPs from the reduction of silver ions. After the tests, the action against the pathogens S. aureus, E. coli, and C. albicans was verified, proving to be effective through a qualitative test. Firoz Babu et al. (2012) incorporated nanoparticles in cotton fabrics. Again, the growth of Escherichia coli and Staphylococcus aureus was inhibited by silver nanoparticles and evaluated through quantitative tests.
Falletta et al. (2008) synthesized AgNPs, incorporated them into cotton, wool, and polyester, and evaluated their antipathogenic effect against S. aureus, S. epidermidis, P. aeruginosa, and C. albicans. The three fabrics showed a good antipathogenic effect. Moreover, it was possible to notice that wool obtained a lower result than the other fabrics due to the low adherence of particles in the textile, which was analyzed by UV–Visible Absorption.
Antimicrobial activity on cotton was reported by Zhang et al. (2009) using silver in the ion form. However, most of the antipathogenic composition on the fabric surface was composed of Ag0. In the study, the silver activity showed a 99.01% and 99.26% reduction against S. aureus and E. coli. The tissues can go through a pre-treatment process to improve some properties. In this case, amino-terminated hyperbranched polymer (HBP-NH2) was added to improve the dyeability and silver fixing, which did not interfere with the antipathogenic action.
Kelly and Johnston (2011) developed a fabric with silver in ion and metal forms. Merino wool was impregnated with AgNPs surrounded with Ag+ that inhibited the microbial growth of S. aureus. In this study, trisodium citrate (TSC) was added as a binder. The increase in TSC concentration improved the incorporation of nanoparticles, consequently achieving a higher antipathogenic action.
Durán et al. (2007) verified the antipathogenic action of ionic silver against Staphylococcus aureus. Cotton fabric loaded with Ag+ was able to reduce 99% of bacteria. Moreover, this study presented an alternative to minimize silver rejection using Chromobacterium violaceum. In this case, biosorption proved efficient, avoiding the remaining silver being released into effluents.
Tomšič et al. (2009) used the pad-dry-cure method with sol–gel containing silver and a binder. The antipathogenic action against the growth inhibition of the pathogens E. coli, A. niger, and C. globosum was evaluated. The sol–gel immersed samples obtained a higher amount of impregnated silver, which improved the performance. In addition, the curing temperature variation was evaluated, but it did not significantly influence the process.
Nevertheless, a preoccupation is the development of super-resistant bacteria, as already reported (Prasher et al. 2018). Some studies have also shown surface alterations or mutations in organisms when exposed to silver (Graves et al. 2015; Kaweeteerawat et al. 2017). Moreover, silver action mechanisms are still under investigation.
Ionic and metallic copper
Another widely used antipathogenic agent is copper, either in metal or ion form as copper oxide, iodide, and silicate. However, copper ions are more common than copper metal, since copper metal can undergo oxidation, so some strategies must be adopted to prevent it (Xu et al. 2018).
Xu et al. (2018) presented an impregnated cotton fabric with copper nanoparticles (CuNPs) during the synthesis of the nanoparticles. The authors used thioglycolic acid (TGA) and citric acid as additives to increase durability and protect the copper from oxidation. The antipathogenic action was evaluated against E. coli and S. aureus after 50 washes. Initially, the samples had a bacterial reduction of 100%, and after the washes, they presented a decrease of 96% for both pathogens.
Sharma et al. (2019) demonstrated the antipathogenic action of CuNPs. The bacterial reduction was 100% and 74% for S. aureus and E. coli, respectively. As in the previous study, the antipathogenic action was evaluated after 50 washes, but, in this case, no binder was added. Therefore, the leaching of the nanoparticles decreased the antipathogenic activity, reaching only 65% and 50% bacterial reduction of S. aureus and E. coli.
An N95 mask with impregnated copper oxide was designed with 4 layers, one of which was a functional layer. The mask was tested against H1N1 and H9N2 viruses, with ~ 3 and ~ 4 log reduction. Furthermore, several mask parameters were tested, such as bacterial filtration efficacy, differential pressure, and resistance to penetration; in all cases, the performance was satisfactory (Borkow et al. 2010).
Shahid et al. developed multifunctional textiles from the incorporation of copper oxide particles, Cu2O. In this work, the particles were synthesized with different reducing agents, which influenced the final antipathogenic potential of each sample. From the three reducing agents, glucose, ascorbic acid and sodium hydrosulphite, the particles produced through the last agent obtained better results in the inhibition of E. coli and S. aureus (Shahid et al. 2021).
The bactericidal potential of copper oxide against S. aureus and E. coli was investigated by El-Nahhal et al. (2012). In this case, CuO nanoparticles were deposited on cotton fibers, and the antipathogenic action demonstrated complete growth inhibition. Vasantharaj et al. (2019) reported an eco-friendly synthesis and evaluated the antibacterial potential of copper oxide. After synthesis, a piece of impregnated cotton fabric was qualitatively tested against bacteria (E. coli, S. aureus, and K. pneumoniae) with satisfactory results.
Titanium dioxide
Studies have also reported the antipathogen activity of titanium dioxide (titania, TiO2), which has a well-known photocatalytic activity. When irradiated, TiO2 produces radicals that help in the antipathogen action. Titania comes in three phases: anatase, rutile, and brookite. As anatase, it presents better photocatalytic performance. Moreover, some studies report the activity in the dark (Galkina et al. 2014).
The antimicrobial activity of facial masks containing titanium dioxide and silver nitrate coating was analyzed against E. coli and S. aureus. Pieces of the facial mask were incubated for 48 h and at the end of the test, a 100% reduction of E. coli and S. aureus was observed (Li et al. 2006).
Galkina et al. (2014) tested TiO2 (anatase and brookite) isolated and deposited on cotton through immersion in suspension with 1,2,3,4-butanetetracarboxylic acid (BTCA), and TiO2. BTCA was used to ensure a better uniformity in the modification of the fiber structure. The modified cotton was evaluated against E. coli and inhibited ~ 70% of microbial growth. Prorokova et al. (2018) modified the surface of polyester by deposition of TiO2 nanoparticles. Pathogen tests under UV light reached inhibition of 31%, 63%, and 83% against E. coli, S. aureus, and C. albicans, respectively.
Polyamide fibers with TiO2 (anatase and rutile) were prepared by electrospinning, and antipathogenic, hydrophilic fibers were obtained (Pant et al. 2011). Moreover, the addition of TiO2 improved some properties like mechanical strength and UV blocking. The antipathogenic activity was evaluated against E. coli under UV light, and inhibition of bacterial growth was shown. Karimi et al. (2014) developed a cotton fabric with antipathogenic properties with the TiO2 as anatase and rutile incorporated by dip coating. The reduction against E. coli, S. aureus, and C. albicans reached > 99%.
Zinc oxide
Like titania, zinc oxide (ZnO) has photocatalytic properties, which are essential for antipathogenic activity. ZnO comes in three phases: wurtzite, zinc blende, and rock salt. Wurtzite is the most stable and common phase.
El-Nahhal et al. (2017) functionalized cotton by a coating of ZnO nanoparticles (wurtzite) and verified the antimicrobial activity against Escherichia coli, Staphylococcus aureus, Candida albicans, and Microsporum canis. In another work, El-Nahhal et al. (2020) obtained a cotton fabric functionalized with ZnO nanoparticles (wurtzite). The impregnation occurred by the sonochemical method using starch as an additive, and the potential against E. coli and S. aureus was evaluated. The samples with only ZnO showed a 100% bacterial reduction for E. coli and S. aureus. After 10 washes, the reduction dropped to 54.9% and 56.9%, respectively. However, the samples with 2% starch showed a 78% and 95% bacterial reduction after washing for E. coli and S. aureus, respectively.
Fiedot-Toboła et al. (2018) modified the surface of several textiles by deposition of ZnO nanoparticles (wurtzite). Polyamide 6 (PA), polyethylene terephthalate (PET), and polypropylene (PP) containing the nanoparticles had a > 99% bacterial reduction for E. coli and S. aureus. The matrix change had no direct influence on the antipathogenic action of ZnO; however, hydrophobic surfaces (PA) showed larger particle clusters than hydrophilic surfaces (PP and PET).
Iron oxide
Ionic iron, both ferrous (Fe2+) and ferric (Fe3+), can also produce radicals that cause damage to membranes and structures of pathogens. Furthermore, magnetic particles can bind more strongly to the pathogen (Harifi and Montazer 2013).
Harifi and Montazer (2013) developed multifunctional fabrics based on polyester, with nanoparticles of either magnetite (Fe3O4) or hematite Fe2O3), which were synthesized in situ. In addition to other properties, the antipathogenic effect of magnetite and hematite against S. aureus reached a reduction of 100% and 80.47%, respectively. Another multifunctional fabric was the subject of the study by Rastgoo et al. (2016) In this case, a 30% cotton/70% polyester composite fabric within situ synthesized magnetite was produced and tested against bacteria and fungi. The bacteria reduction was 95% against S. aureus, and the fungus reduction was 99% against C. albicans.
The inactivation of the influenza virus by Fe3O4 was compared to the performance of peroxidase and catalase enzymes. Magnetite was incorporated into facial masks in different concentrations (0.8, 0.4, 0.2, and 0.1 mg/cm2) and incubated with H1N1 viruses, H5N1, and H7N9. TCID50 and hemagglutinin activities were measured, and levels were evaluated at 0.5 and 1 h. The application with 0.8 mg/cm2 of iron oxide inactivated the virus present in the mask within 30 min (Qin et al. 2019).
Processing: incorporating antipathogenic agents into fabrics
The application of particles in fabrics can be made in several ways, along with the manufacturing process of fibers and textiles (Schindler and Hauser 2004; Gao and Cranston 2008). Functionalized substances can be obtained through chemical and physical processes. The antipathogenic agent is usually incorporated in the last processing stage of wet-processing, i.e., finishing, as seen in Fig. 4. Alternatively, it is also possible to integrate the antipathogenic agents during the formation of the fibers, i.e., fiber preparation, as also shown in Fig. 4, which provides a fabric with better durability of antipathogenic effect (Gao and Cranston 2008).
Dip-coating, pad-dry-cure, and sonication are widely used methods, which consist of a direct application of a colloidal dispersion containing the particles, through the immersion of the tissue in the dispersion. Details on each process are discussed below.
Dip coating
Dip coating is a simple and easy method to apply but does not provide a uniform coating. On the other side, this process can be used in fibers or fabrics and causes no damage or distortions to the fabric or fibers (Joshi and Butola 2013). It consists of immersing the fabric in a suspension (Fig. 5), with or without removing the excess fluid. Therefore, the materials (antipathogenic agents) are dispersed in solutions that will coat a surface (fabric), then the fabric is put to dry. Many parameters can influence the results, such as viscosity, immersion time, speed, surface tension, etc. (Tang and Yan 2017).
Dip coating was used by Kumar (2016), in which a piece of cotton fabric was immersed in a suspension containing the silver particles for 1 h and then dried at 600 °C. Lin et al. (2015) used the dip-coat technique to create a superhydrophobic surface. The cotton textile was immersed in a dispersion containing DDS-SiO2 particles in DMF for 5 min and dried at 160 °C for 5 min to remove the solvent. A second dip coating was done, 10 wt% fluoropolymers in DMF, and the immersion and dry process was repeated. This process allows the textile to have low water/oil absorption ability and self-cleaning properties (Lin et al. 2015).
Pad-dry-cure
In pad-dry-cure (Fig. 6), the woven textile gets immersed in a dispersion, and then it is passed between cylinders, which ensures a homogeneous distribution in the fabric, provides a good fixation and has a simple system (Gulrajania and Gupta 2011). After the immersion, the crosslinking reactant, catalyst, softener, and other components are dried on the fabric. Finally, a crosslinking reaction takes place during the curing step.
Hasan (2018) immersed cotton fabric in a solution containing CuO and binder for 5 min, then passed through a padding mangle. After drying naturally, it was cured for 3 min at 140 °C. In general, this is a simple methodology and provides a uniform coating but requires the use of a binder.
Yadav et al. (2006) also applied the same method, but in a solution containing ZnO for 5 min and then removed the excess solution through a padding mangle After drying and curing, the woven fabric with impregnated ZnO presented UV blocking properties. Kangwansupamonkon et al 2009. used the pad-dry-cure technique to coat a cotton textile with apatite/titanium dioxide (TiO2). The cotton was immersed in a dispersion containing apatite/titanium dioxide (TiO2), then was dried at 100 °C for 5 min and cured at 150 °C for 3 min. After this process, the cotton textile presents antimicrobial properties (Kangwansupamonkon et al. 2009).
Cotton fabrics with antimicrobial, UV-protective, and self-cleaning properties were prepared by Onar et al. (2011) The fabrics were dipped in a solution of AgNO3, titanium isopropoxide, and tetraethyl orthosilicate, for 30 s. Then, they were dried at 80 °C for 30 min and cured at 150 °C for 5 min.
Sonochemical coating
The sonochemical coating is one of the best methods from the point of view of adhesion and uniformity, which enhances the durability and antipathogenic effect, but it is more expensive than the methods previously mentioned. In this method, the fabric is submitted to sonication in a dispersion, then it is dried (Fig. 7).
Perkas et al. (2018) synthesized AgNPs within cotton. The fabrics were then submitted to high-intensity ultrasound for 1 h, and finally washed and dried. This technique was also applied to the coating of cotton with CuO by Abramova et al. (2009), and cotton with ZnO by Noman and Petrů (2020), among others.
The study presented by Abramov et al. (2013) involves the development of a pilot-scale sonochemical coating and produced a biocidal fabric. To use this method is necessarily a solution, which contains copper acetate monohydrate, water, and ethanol. A cotton bandage was submitted to sonication, the temperature reached approximately 60 °C, and then dried under vacuum.
This technique was used by Petkova et al. (2014), in a simple procedure, the cotton fabric was added in a dispersion composed of ZnO NPs, chitosan, and water. The ultrasound was performed for 30 min, then the fabric was washed and dried. The textile presented antimicrobial properties.
Spraying
Spraying is another simple method, an easy application system, and this makes it possible to use it on several surfaces including fabrics. The dispersion is forced through a nozzle, and an aerosol is formed and coats a surface (Fig. 8). The coated parameters are the concentration, spray time, diameter, nozzle pressure, and others. A reapplication is possible, but this can cause a non-homogeneous coating (Joshi and Butola 2013).
A spray can cover a non-woven surgical mask with copper nanoparticles, conferring an antimicrobial action and enabling the reuse of the mask (Kumar et al. 2020). Balagna et al. (2020), used radio frequency to apply silver into an FFP3 mask at two different powers, which reached increasing amounts of metal coverage. Later, the antiviral action of silver against SARS-CoV-2 was verified. Latthe et al. (2019) sprayed a cotton shirt coat with silica nanoparticles. The NPs were dispersed in hexane, sprayed in the textile, and then evaporated to obtain a hydrophobic surface.
A superhydrophobic/superhydrophilic cotton fabric was produced by Sasaki et al. (2016) The method used was spraying coating, a dispersion of acetone with SiO2 nanoparticles, and ethyl-α-cyanoacrylate. After being sprayed, the cotton fabric was dried at room temperature. In this study, the author varied the spraying distance: in a short distance, a hydrophobic surface was obtained; in a long distance, a hydrophilic surface was achieved; and in a medium distance, one side was superhydrophobic and the other side, superhydrophilic.
Electrospinning
Electrospinning can be explored to manufacture non-woven textiles (Fig. 9). It allows particles to be added to the solution, which provides functionality to the tissue, but this method has hindrances for application on a large scale. Electrospinning offers suitable control of the structure and properties of the textile. To form an antipathogenic fabric, two different incorporation methodologies are generally adopted: either the functional particles are in the solution to be electrospun, or a dispersion containing functional particles is added to the fiber surfaces after electrospinning.
Tijing et al. (2012) fabricated tourmaline NPS/polyurethane composite by electrospinning. First, a polyurethane and tourmaline solution was prepared in DMF. Then, the solution was electrospun to form a non-woven fabric, which presented antibacterial and superhydrophilic properties. Hwang and Jeong (2011) investigated three different solutions containing poly(vinyl pyrrolidone) and AgNPs, varying the size of the nanoparticles. The best results obtained an effective antimicrobial fiber against S. aureus, K. pneumoniae, and E. coli.
A material with antibacterial, antiviral, and self-cleaning properties was fabricated by Karagoz et al. (2021). In this work, a DMF dispersion was prepared to contain ZnO nanorods, Triton X-100 (surfactant), PMMA, and AgNO3. Using the electrospinning method, it was possible to construct nanofibers that can apply to protective clothing.
An example of the surface modification to turn functional is presented by Ranjbar-Mohammadi and Yousefi (2021). The 3D fabrics were modified by TiO2/Nylon‑6 nanofibers produced by electrospinning. This process improved some properties allowing the use in a dye removal system.
Evaluation of antipathogenic effect
Antipathogenic mechanism
The antipathogenic action of particles or ions may be explained by different mechanisms. In some cases, particles adhere to the membrane by electrostatic forces, and in other cases, they break the membrane causing intracellular damage and suffering oxidative processes (Rai et al. 2014; Deshmukh et al. 2019). In addition, particles can compete in the connection with the cells and break the pathogen cover (Mehrbod et al. 2009; Rai et al. 2014; Salleh et al. 2020). Some pathogens use the angiotensin-converting enzyme 2 (ACE2) receptor, which is present in cells in several human tissues. Thus, the human receptor and the spike protein interact powerfully (Fig. 10), creating the link between them and causing the host infection (Naqvi et al. 2020; Shang et al. 2020; Walls et al. 2020; Wang et al. 2020a). Some mechanisms regarded as responsible for the action against bacteria and viruses are summarized in Table 2.
Some antipathogenic agents, such as metallic silver, have major mechanisms related to avoiding the connections between the proteins of the virus with the cellular receiver. This occurs due to the destruction of proteins such as hemagglutinin and spike which includes the lipid layer that surrounds the virus. For the contamination to occur in host cells, it is necessary the interaction of the spike proteins with the ACE2 cellular receptor. Therefore, with the antipathogenic agents, the interaction is blocked, preventing the entrance of the pathogens, (Gao et al. 2017; Qin et al. 2019; Salleh et al. 2020) as represented in Fig. 10. According to Elechiguerra et al. (2005), AgNPs form bonds with glycoprotein, producing an envelope wrapped in the virus that prevents its binding with other cells. Furthermore, Lara et al. (2010b) and Morris et al. (2019) demonstrated that the action of AgNPs interfered with gp120-CD4 interaction, inhibiting the action of HIV-1 and RSV virus.
As already mentioned, in other cases involving ions, the proteins and lipid layer deterioration, such as found in bacteria, viruses, and fungi, can be caused by reactive oxygen species (ROS), as shown in Fig. 11, which are generated from catalytic processes that occur with antipathogenic agents. The intermediates generated as hydroxyl free radicals (OH−), hydroperoxyl HO2−, and superoxide O2−, among others, capture an H+ from a hydrogen donor substance, such as proteins, lipids, polysaccharides, and nucleic acids (Gao et al. 2007, 2017; Anjum et al. 2015).
Antipathogenic activity
To obtain functionalized fabrics it is important to follow some standards for measuring the antipathogenic activity, as detailed in Table 3.
The methods described evaluate the growth of the pathogen and are comparison tests between treated and untreated fabrics. Assay selection should consider the type of antipathogenic agent, woven/non-woven textile so that the most appropriate standard is chosen, as detailed below.
Antibacterial activity
Tests to evaluate the antibacterial properties generally include inhibition tests against Staphylococcus aureus (gram-positive) and Escherichia coli or Klebsiella pneumoniae (gram-negative) (Simončič and Tomšič 2017). The most common and simple method uses a plaque with agar, which is infected with some bacteria containing a treated fabric and a control fabric. After an incubation period, it is possible to identify the area where no bacterial growth has occurred (Fig. 12). The larger the area, the better the antibacterial effect. However, this test is more likely to be used with antibacterial leaching agents. If it is a non-leaching agent, it is necessary to compare it to the control test. Another assay that can be used involves counting bacterial colonies in different incubation periods. Since this is a more quantitative assay, the count can be done visually or using the adenosine triphosphate (ATP) method (Pankaj 2013).
Some tests are performed to determine the minimum inhibitory concentration (MIC) to verify the antimicrobial activity. They can be conducted in liquid or solid growth medium plates. MIC is defined as the lowest concentration of a substance that will inhibit the visible growth of a pathogen during a specific incubation period, i.e., the lower the MIC, the smaller the quantity of substance needed to inhibit growth (Jennifer 2001; Biology LibreTexts 2021).
Antiviral activity
For antiviral activity evaluation, ISO 18184:2019 (International Organization for Standardization 2019) standard suggests two assays: plaque or TCID50 method, against influenza A virus (H1N1 or H3N2) and/or feline calicivirus.
The plaque assay (Fig. 13) is a simple technique, in which dilutions of stock viruses are prepared. The virus previously contacted the functionalized fabric and the control fabric. Aliquots are added to plates containing a medium and cells. An incubation time is required for cell infection. After this period, the host cells are covered with a semi-solid substrate to prevent the virus from infecting other cells and to perform the count.
In the TCID50 method, several dilutions are performed, and the concentrations of the pathogen are measured. Then, the infected cells are incubated for 7 days, enough for cytopathic effects (CPE), which allows for analyzing the infected cells of each dilution. By using a microscope and statistical methods, such as Behrens and Karber analysis, the concentration can be calculated.
Other techniques can be used, such as immunofluorescence Foci Assay (IFA), to quantify the virus concentration. This method uses antibody-based staining substances and therefore can be employed in cases where plaque formation is not supported or does not have CPE (Pankaj 2013; Krumm 2020).
Alternatively, the polymerase chain reaction (PCR) enables the detection of the pathogens' nucleic acid. In this case, a probe binds to a specific region of DNA and emits a detectable signal. RNA can also be detected as long as there is a reverse transcription from RNA to DNA (RT-PCR) (Pankaj 2013; Krumm 2020).
Finally, a hemagglutination assay is applied when pathogens contain several proteins that can bind and form agglutination of antibodies or red blood cells. Thus, if the pathogen is present, agglutination occurs; otherwise, the result is negative (Pankaj 2013; Krumm 2020; Lumen).
Antifungal activity
Most of the methods to detect antifungal activity are visual. They are applied against Aspergillus niger and Chaetomium globosum and performed by inoculating the fungi on agar plates (Simončič and Tomšič 2017). As reported in the antibacterial tests, the assays must contain a control with a fabric without an antipathogenic agent and another containing the functional fabric. After an incubation time, it will be possible to check the fungal growth and the intensity of the medium in contact with the fabric.
Another method of antifungal determination is presented by ISO 13629-2:2014 (International Organization for Standardization 2014), which consists of counting or ATP luminescence. A suspension containing the fungus and the fabrics are incubated for a period, and then the activity is determined quantitatively.
Properties and performance of antipathogenic fabrics
In this case, the antipathogenic activity is the major property that the tissue can exhibit. Nevertheless, a set of properties gives the fabric a unique performance, which allows varying the properties and consequently its application. Mechanical properties, durability, breathability, electrostatic, and fire-retardant, among others, can be combined to produce the desired fabric.
Life cycle and durability
Fabrics can be incorporated with antipathogenic particles that have an adherence and fixation in the fibers, which can be shorter or longer. This treatment can be performed with binders such as dimethyloldihydroxyethylene urea (DMDHEU), polyurethane resin, and polyacrylic esters (PALS), and also through pre-treatments involving bio and plasma technologies (Zhang et al. 2009; Abdel-Aziz et al. 2014; Ibrahim et al. 2017b, 2022; Naebe et al. 2022).
Zhang et al. (2009) used a dye fixative (HBP-NH2) to help sustain the antipathogenic effects in cotton. After 20 washes, there was no significant change in the initially reported inhibition, which was greater than 98.77% against S. aureus and E. coli. Another similar study evaluated the importance of using a binder for particle adhesion. First, silver-coated cotton fabrics were tested against S. aureus and E. coli, which were reduced by 97% and 91%, respectively. The fabric was subjected to 20 washes and retested, achieving a significant bacterial reduction of 53% and 48.7%. In a second test, the cotton fabric was treated with a binding agent, and after washing, a bacterial reduction of 94% and 85% was achieved, proving that using a binder was important for the optimization and durability of the antipathogenic effect (El-Rafie et al. 2010).
El-Nahhal et al. (2017) tested some surfactants to fix ZnO nanoparticles in cotton, such as sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), Triton X-100 (TX-100), and alkyl-hydroxyethyl dimethylammonium chloride (HY). The fabric treated with HY performed better, both in the first test and in the test after 10 washes, and showed a reduction of more than 90% against all pathogens, a difference of about 1–1.5%. The SDS treatment had a small difference, about 2%. CTAB and TX-treated, and the untreated sample had a significant difference of about 7%.
In the work presented by Ibrahim et al., finishing baths containing citric acid (CA) or succinic acid (SA) were applied on cotton/wool (C/W) and viscose/wool (V/W) fabrics. The finishing bath also contained Ag and/or ZnO nanoparticles. This finishing with CA or SA improved the antipathogenic effect, besides helping in the fixation of the nanoparticles, which maintained their performance after 10 washes. Ibrahim et al. reported in other works the use of enzymes, carboxymethylation and plasma as pre-treatment (Abdel-Aziz et al. 2014; Ibrahim et al. 2017a, 2018, 2022).
Salat et al. (2018) treated cotton sonochemically with zinc oxide and gallic acid (GA). In this work, a sonochemical coating was used, with a suspension containing ZnO, GA, and laccase. The laccase and GA form a polymer, fixing better ZnO. Figure 14 shows the importance of activation promoted by laccase. Even after 60 washes, the antipathogenic effect showed a 60% bacterial reduction.

Adapted from Salat et al. (2018)
A Antibacterial activity of the fabrics sono-enzymatically coated with ZnO after multiple washes at 75 °C and B amount of ZnO remaining on the fabrics after multiple washes at 75 °C.
Another way that can modify the durability of the antipathogenic effect is through the technique used to incorporate the agents into the fabric.Firdhouse and Lalitha (2013) used dipping, and sonication methods to modify a cotton fabric. Sonication offered a better uniformity and amount of antipathogenic agents. Consequently, this improved the antipathogenic effect compared to immersion.
Biodegradation
Some microorganisms cause deterioration effects in the fabrics, damaging them gradually and leading them to degradation. It is important to emphasize that this only slows down the degradation, increasing their durability, and does not contaminate the soil (Teufel and Redl 2006; Malis et al. 2019).
A study conducted with nanoparticles incorporated in cotton and cotton/polyester (El-Naggar et al. 2003), several concentrations of ZnO and their influence on some mechanical properties were evaluated, before and after a biodegradation or soil burial test. Initially, the concentrations of binder and dispersant were preserved, and there was variation only in the concentration of ZnO. For the tensile strength and elongation test before burial, the application of ZnO increased the strength and elongation of the fabric in relation to the untreated textile. The author believes that this is due to the small surface layers formed with the coating. After one week on the ground, the textiles were evaluated again, and there was a reduction in tensile strength and elongation. Still, a better result was obtained when compared to the fabric without ZnO. After 2 weeks, the fabric composed only of cotton without coating was worn out, and the test could not be carried out. Therefore, the ZnO protected the fabric against the action of microbes, delaying the decomposition.
Mechanical properties
When antipathogenic agents are incorporated into textiles, doubts have arisen about the degradation of mechanical properties, as many of these agents use ROS, and this mechanism could also affect the fabric.
In a recent paper, Tania and Ali (2021) carried out tests to evaluate some mechanical properties of fabric after the introduction of an antipathogenic agent. Using the pad dry cure, cotton was covered with ZnO nanoparticles to obtain a fabric with anti-pathogenic properties. In this case, it was tested against E. coli and S. aureus resulting in a reduction greater than 98% and 97%, respectively, and the mechanical properties were improved. In a first test, the crease resistance was evaluated, and the recovery in the angle was evaluated in untreated fabric and treated with 1% ZnO, 1% ZnO + binder, or 1% ZnO + binder + polyethylene wax. Compared to the untreated sample, treated samples presented an improved crease recovery of 3%, 5.7%, and 10%, respectively. Untreated samples treated with 1% ZnO only had a significant reduction in tensile strength, about 5.6%, and 3.9%, for warp and weft directions, respectively. In the sample with 1% ZnO only, the reductions were 10.5% (warp) and 12.2% (weft). In sample 1% ZnO + binder, there was an increase in tensile strength, 8.8%, and 7.6% in warp and weft directions, respectively. Thus, ZnO reduces the tensile strength of cotton when used alone. However, when polyethylene wax emulsion was added, an improvement was observed due to a polymeric bonding. For elongation and tearing strength tests, the results showed the same trend. Besides the properties mentioned, the coating with ZnO caused an increase in the stiffness of the fabrics, except for the sample with 1% ZnO + binder + polyethylene wax, in which the stiffness was reduced by ~ 22% in relation to the untreated fabric due to the softness caused by the wax emulsion (Tania and Ali 2021).
Safety and environmental issues
One of the concerns about textiles that contain antipathogenic particles is the impact of leaching into the environment. It is expected that fabrics release these particles as the physical and/or chemical finishing techniques that are used to incorporate them can be reversible.
Some studies on antipathogenic agents have reported releasing of contaminants. As already mentioned, silver in contact with microorganisms can cause genetic changes and poses danger to natural and necessary microorganisms. Graves et al. (2015) performed a genetic study related to the resistance of E. coli bacteria to AgNPs. The genomic analyses showed the bacterium evolution and the development of resistance to AgNPs, through successive mutations. Kaweeteerawat et al. (2017) pre-exposed E. coli and S.aureus to a sublethal dose AgNPs, these microorganisms increased the resistance toward antibiotics (ampicillin and Pen-Strep). Furthermore, in the sample pretreated the membrane damage and oxidative stress decreased, suggesting AgNps can stimulate mutations.
TiO2 occurs in large quantities in wastewater, especially in sediments. Because of its low solubility, it becomes more resistant (Tourinho et al. 2012; Simončič and Tomšič 2017). When leached, titania nanoparticles can be released into aquatic environments and be harmful to organisms essential to the ecosystem. Valério et al. (2020) investigated potential harms caused by TiO2 nanoparticles using the embryonic development of zebrafish (Danio rerio) as a proxy. Acute sublethal effects were recorded in zebrafish embryos at different stages of development, related to the release of TiO2 nanoparticles into the aquatic environment (Fig. 15).

Adapted from Valério et al. (2020)
Structural defects, such as yolk deformations, pericardial edemas, arched tails, and others, can be observed in the developmental traits of zebrafish larvae induced by TiO2.
Other studies (Andy et al. 2008; Bondarenko et al. 2013) report that some species are affected by silver, ZnO, and CuO. In comparison, iron oxide seems to present lower toxicity towards the bacteria Shewanella oneidensis. Nevertheless, if necessary, prevention techniques can be incorporated into water treatment. In the case of silver, it can be absorbed by a fungus (C. violaceum) (Durán et al. 2007), for example.
In addition, if the fabrics have been modified, so their behavior will be different, which can delay their decomposition. The natural degradation of fibers or textiles is caused by microorganisms, which can suffer from the antipathogenic effect of the agents. Tourinho et al. (2012) presented the effects caused by some antipathogenic agents on soil organisms. Of the compounds reported, TiO2 was shown to be less toxic than Ag and ZnO against soil organisms such as Caenorhabditis elegans, Eisenia fétida, and Porcellio scaber. In the soil, these agents can still influence the crops, as in the case of ZnO decreasing the germination and growth of some plants, and TiO2 in high doses can be phytotoxic (Andy et al. 2008).
When these compounds are available in the environment, consumption and contact with animals and humans are possible. Moreover, during the use of textiles, antipathogenic agents can pose risks to the user's skin and penetrate and damage cells (Simončič and Tomšič 2017). For instance, Ingle et al. (2013) reported CuO nanoparticles can penetrate the human skin, be inhaled, or digested, and cause respiratory, gastrointestinal, and other problems. However, it is worth noting that a high concentration of metal ions or nanoparticles is required to cause problems in humans. Furthermore, as mentioned, it is possible to increase the fixation of the particles to the fabric, making the release more difficult and reducing the environmental impact.
Commercial applications and perspectives
Since the mid-1950s, there have been patent applications for woven or non-woven fibers and textiles with antipathogenic properties (antifungal or antimicrobial). In these applications, several antipathogenic agents have been exploited, such as silver, silver nitrate, copper, copper oxide, zinc oxide, iron oxide, titanium dioxide, polyphenol, chitosan, triclosan, and carbon nanotubes, among others. These textiles can be applied in clothing, protective equipment such as masks, gloves, aprons, health care items, households, and similar.
Lau et al. (2018) developed cellulosic and non-cellulosic antimicrobial fabrics that showed durability after more than 100 washes. The authors incorporated several types of cationic biocide via a pad-dry-cure method and obtained a > 99.9% reduction against Staphylococcus aureus and Klebsiella pneumoniae before and after 104 washes.
Two patents focusing on attractive applications were published recently (Gabbay 2008, 2016). In a case, after the harvest, fruits such as strawberries, are susceptible to attacks by microorganisms, so the idea of developing a polymeric fabric with antimicrobial and antiviral properties for food packaging came up. The polymeric blend was investigated in three situations against HIV-1, (1) Polymeric Fiber without CuO and Cu2O. (2) CuO and Cu2O powder and (3) Fiber impregnated with 1% CuO and Cu2O. These samples obtained the following results: no inhibition, 70% inhibition, and 26% inhibition, respectively. The antibacterial and antifungal tests exhibited an inhibitory zone, indicating the effect of biocide.
Another application for commercial use was presented by Sahin et al. (2017), with hygienic purposes. The invention is based on woven or non-woven fabrics with antipathogenic, and hydrophilic properties, for sanitary pads, bladder pads, tampons, and diapers. The development of microorganisms is favorable due to the conditions of use, so this technology can prevent diseases caused by bacteria, fungi, and viruses.
The application of antipathogenic fabrics is not limited to just one property. Other properties, such as UV blocking, self-cleaning, hydrophobicity, and others, can be obtained and combined. This raises the expectation of growth, added to the high demand for antipathogenic materials. Moreover, the problems with particle leaching can be controlled through different methodologies used, minimizing impacts on the environment.
Conclusions
Textile functionalization can be promising for fibers and textiles, as the current demand for antipathogenic products is expected to increase in the coming years. Metal oxides and metals present stability and compatibility and are widely in demand due to their antipathogenic potential.
Various types of fabrics and processing techniques can be combined to produce antipathogenic woven or non-woven textiles. Thus, a wide variety of fabrics can be manufactured with the desired properties for each application. Cellulose fabrics provide biodegradability and are more eco-friendly, the versatility and properties of this fabric lead to large production and consumption.
However, it is important to choose processing techniques that optimize the antipathogenic agents' fixation in the fabric. Some compounds can favor resistance to substances used in the medicinal environment. The incorporation of agents during finishing can impair fixation. Studies have shown that sonochemical techniques offer better adhesion than dip-coating, for instance. If possible, agents should be incorporated during fiber formation, offering better fixation and consequently less leaching to the environment, and the use of binders should be considered.
There is a growing demand for antipathogen tissues, and the variety of agents and tissues leads to diverse applications, thus increasing their use even more. Therefore, investigations on antipathogenic properties of various compounds are becoming more and more common. However, there is also a need to step up studies of health and environmental effects and explore less harmful antipathogenic agents and processes.
References
American Association of Textile Chemists and Colorists (2011) AATCC 147: 2011 test method for antibacterial activity of textile materials: Parallel Streak
American Association of Textile Chemists and Colorists (2012) AATCC 100:2012 Antibacterial Finishes on Textile Materials
Abdel-Aziz MS, Eid BM, Ibrahim NA (2014) Biosynthesized silver nanoparticles for antibacterial treatment of cellulosic fabrics using O2-plasma. AATCC J Res 1:6–12
Abramov OV, Gedanken A, Koltypin Y, Perkas N, Perelshtein I, Joyce E, Mason TJ (2009) Pilot scale sonochemical coating of nanoparticles onto textiles to produce biocidal fabrics. Surf Coat Technol 204:718–722. https://doi.org/10.1016/J.SURFCOAT.2009.09.030
Abramova A, Gedanken A, Popov V, Ooi EH, Mason TJ, Joyce EM, Beddow J, Perelshtein I, Bayazitov V (2013) A sonochemical technology for coating of textiles with antibacterial nanoparticles and equipment for itsimplementation. Mater Lett 96:121–124. https://doi.org/10.1016/J.MATLET.2013.01.041
Ali A, Baheti V, Militky J, Khan Z, Tunakova V, Naeem S (2018) Copper coated multifunctional cotton fabrics. J Ind Text 48:448–464. https://doi.org/10.1177/1528083717732076
Ali A, Hussain F, Attacha S, Kalsoom A, Qureshi WA, Shakeel M, Militky J, Tomkova B, Kremenakova D (2021a) Development of novel antimicrobial and antiviral green synthesized silver nanocomposites for the visual detection of Fe3+ ions. Nanomaterials 11:2076. https://doi.org/10.3390/nano11082076
Ali A, Hussain F, Kalsoom A, Riaz T, Zaman Khan M, Zubair Z, Shaker K, Militky J, Noman MT, Ashraf M (2021b) Multifunctional electrically conductive copper electroplated fabrics sensitizes by in-situ deposition of copper and silver nanoparticles. Nanomaterials 11:3097. https://doi.org/10.3390/nano11113097
American Association of Textile Chemists and Colorists (2016) AATCC 90:2016 test method for antibacterial activity of textile materials: Agar Plate
American Association of Textile Chemists and Colorists (2017) AATCC 30:2017 test method for antifungal activity, assessment on textile materials: Mildew and Rot Resistance of Textile Materials
Andra S, Balu SK, Jeevanandam J, Muthalagu M (2021) Emerging nanomaterials for antibacterial textile fabrication. Naunyn Schmiedebergs Arch Pharmacol 394:1355–1382. https://doi.org/10.1007/s00210-021-02064-8/Published
Andy RIDH, Yon DEYL, Ahendra SHM, Aughlin MIJMCL, Ead JARL (2008) Critical review and effects. Environ Toxicol Chem 27:1825–1851. https://doi.org/10.1897/08-090.1
Anjum NA, Sofo A, Scopa A, Roychoudhury A, Gill SS, Iqbal M, Lukatkin AS, Pereira E, Duarte AC, Ahmad I (2015) Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res 22:4099–4121. https://doi.org/10.1007/s11356-014-3917-1
Balagna C, Perero S, Percivalle E, Nepita EV, Ferraris M (2020) Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram 1:100006. https://doi.org/10.1016/j.oceram.2020.100006
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200. https://doi.org/10.1007/S00204-013-1079-4
Borkow G, Zhou SS, Page T, Gabbay J (2010) A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 5:e11295. https://doi.org/10.1371/journal.pone.0011295
Biology LibreTexts (2021) Minimal inhibitory concentration (MIC). https://bio.libretexts.org/@go/page/11954?pdf. Accessed 30 Aug 2021
Das B, Imran Khan M, Jayabalan R, Behera SK, Yun S-I, Tripathy SK, Mishra A (2016) Understanding the antifungal mechanism of Ag@ZnO core-shell nanocomposites against Candida krusei. Sci Rep 6:1–12. https://doi.org/10.1038/srep36403
Deshmukh SP, Patil SM, Mullani SB, Delekar SD (2019) Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C 97:954–965. https://doi.org/10.1016/j.msec.2018.12.102
Durán N, Marcato PD, De Souza GIH, Alves OL, Esposito E (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol 3:203–208. https://doi.org/10.1166/jbn.2007.022
Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ (2005) Interaction of silver nanoparticles with HIV-1. J Nanobiotechnology 3:1–10. https://doi.org/10.1186/1477-3155-3-6
El-Naggar AM, Zohdy MH, Hassan MS, Khalil EM (2003) Antimicrobial protection of cotton and cotton/polyester fabrics by radiation and thermal treatments. I. Effect of ZnO formulation on the mechanical and dyeing properties. J Appl Polym Sci 88:1129–1137. https://doi.org/10.1002/APP.11722
El-Naggar ME, Khattab TA, Abdelrahman MS, Aldalbahi A, Hatshan MR (2020) Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation. Cellulose 28:1105–1121. https://doi.org/10.1007/S10570-020-03537-4
El-Nahhal IM, Zourab SM, Kodeh FS, Selmane M, Genois I, Babonneau F (2012) Nanostructured copper oxide-cotton fibers: synthesis, characterization, and applications. Int Nano Lett 2:1–5. https://doi.org/10.1186/2228-5326-2-14
El-Nahhal IM, Manamah AER, Al Ashgar NM, Amara N, Selmane M, Chehimi MM (2017) Stabilization of nano-structured ZnO particles onto the surface of cotton fibers using different surfactants and their antimicrobial activity. Ultrason Sonochem 38:478–487. https://doi.org/10.1016/J.ULTSONCH.2017.03.050
El-Nahhal IM, Salem J, Anbar R, Kodeh FS, Elmanama A (2020) Preparation and antimicrobial activity of ZnO-NPs coated cotton/starch and their functionalized ZnO-Ag/cotton and Zn(II) curcumin/cotton materials. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-61306-6
El-Rafie MH, Mohamed AA, Shaheen TI, Hebeish A (2010) Antimicrobial effect of silver nanoparticles produced by fungal process on cotton fabrics. Carbohydr Polym 80:779–782. https://doi.org/10.1016/J.CARBPOL.2009.12.028
Emam HE (2019) Antimicrobial cellulosic textiles based on organic compounds. 3 Biotech 9:29. https://doi.org/10.1007/s13205-018-1562-y
Falletta E, Bonini M, Fratini E, Nostro AL, Pesavento G, Becheri A, Nostro PL, Canton P, Baglioni P (2008) Clusters of poly(acrylates) and silver nanoparticles: structure and applications for antimicrobial fabrics. J Phys Chem C 112:11758–11766. https://doi.org/10.1021/JP8035814
Felgueiras C, Azoia NG, Gonçalves C, Gama M, Dourado F (2021) Trends on the cellulose-based textiles: raw materials and technologies. Front Bioeng Biotechnol 9:1
Fiedot-Toboła M, Ciesielska M, Maliszewska I, Rac-Rumijowska O, Suchorska-Woźniak P, Teterycz H, Bryjak M (2018) Deposition of zinc oxide on different polymer textiles and their antibacterial properties. Materials 11:707. https://doi.org/10.3390/MA11050707
Firdhouse MJ, Lalitha P (2013) Fabrication of antimicrobial perspiration pads and cotton cloth using Amaranthus dubius mediated silver nanoparticles. J Chem 2013:741743. https://doi.org/10.1155/2013/741743
Firoz Babu K, Dhandapani P, Maruthamuthu S, Anbu Kulandainathan M (2012) One pot synthesis of polypyrrole silver nanocomposite on cotton fabrics for multifunctional property. Carbohydr Polym 90:1557–1563. https://doi.org/10.1016/J.CARBPOL.2012.07.030
Gabbay J (2008) Polymeric master batch, processes for producing polymeric materal therefrom and products produced therefrom
Gabbay J (2016) Antimicrobial and antiviral polymeric material
Galkina OL, Sycheva A, Blagodatskiy A, Kaptay G, Katanaev VL, Seisenbaeva GA, Kessler VG, Agafonov AV (2014) The sol–gel synthesis of cotton/TiO2 composites and their antibacterial properties. Surf Coat Technol 253:171–179. https://doi.org/10.1016/J.SURFCOAT.2014.05.033
Gao Y, Cranston R (2008) Recent advances in antimicrobial treatments of textiles. Text Res J 78:60–72. https://doi.org/10.1177/0040517507082332
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583. https://doi.org/10.1038/nnano.2007.260
Gao L, Fan K, Yan X (2017) Iron oxide nanozyme: a multifunctional enzyme mimetic for biomedical applications. Theranostics 7:3207–3227. https://doi.org/10.7150/thno.19738
Graves JL, Tajkarimi M, Cunningham Q, Campbell A, Nonga H, Harrison SH, Barrick JE (2015) Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front Genet. https://doi.org/10.3389/FGENE.2015.00042
Gulati R, Sharma S, Rakesh KS (2021) Antimicrobial textile: recent developments and functional perspective. Polym Bull. https://doi.org/10.1007/s00289-021-03826-3
Gulrajania ML, Gupta D (2011) Emerging techniques for functional finishing of textiles. Indian J Fibre Text Res 36:388–397
Harifi T, Montazer M (2013) In situ synthesis of iron oxide nanoparticles on polyester fabric utilizing color, magnetic, antibacterial and sono-Fenton catalytic properties. J Mater Chem B 2:272–282. https://doi.org/10.1039/C3TB21445A
Hasan R (2018) Production of antimicrobial textiles by using copper oxide nanoparticles. Int J Contemp Res Rev 9:20195–20202
Hwang S, Jeong S (2011) Electrospun nano composites of poly(vinyl pyrrolidone)/nano-silver for antibacterial materials. J Nanosci Nanotechnol 11:610–613. https://doi.org/10.1166/jnn.2011.3243
Ibrahim NA, El-Zairy EM, Eid BM, Emam E, Barkat SR (2017a) A new approach for imparting durable multifunctional properties to linen-containing fabrics. Carbohydr Polym 157:1085–1093. https://doi.org/10.1016/j.carbpol.2016.10.074
Ibrahim NA, Nada AA, Hassabo AG, Eid BM, Noor El-Deen AM, Abou-Zeid NY (2017b) Effect of different capping agents on physicochemical and antimicrobial properties of ZnO nanoparticles. Chem Pap 71:1365–1375. https://doi.org/10.1007/s11696-017-0132-9
Ibrahim NA, Emam E-AM, Eid BM, Tawfik TM (2018) An eco-friendly multifunctional nano-finishing of cellulose/wool blends. Fibers Polym 19:797–804. https://doi.org/10.1007/s12221-018-7922-8
Ibrahim NA, Eid BM, Sharaf SM (2019) Functional finishes for cotton-based textiles: current situation and future trends. In: Textiles and clothing. Wiley, New York, pp 131–190
Ibrahim NA, Eid BM, Fouda MMG (2021) Chapter 29—the potential use of nanotechnology for antimicrobial functionalization of cellulose-containing fabrics. In: Ibrahim N, Hussain CM (eds) Green chemistry for sustainable textiles. Woodhead Publishing, pp 429–451
Ibrahim NA, Amin HA, Abdel-Aziz MS, Eid BM (2022) A green approach for modification and functionalization of wool fabric using bio- and nano-technologies. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-022-02385-z
Ingle AP, Duran N, Rai M (2013) Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol 98:1001–1009. https://doi.org/10.1007/S00253-013-5422-8
International Organization for Standardization (2004) ISO 20645:2004 Textile fabrics—determination of antibacterial activity—Agar diffusion plate test
International Organization for Standardization (2013) ISO 20743:2013 Textiles—Determination of antibacterial activity of textile products
International Organization for Standardization (2014) ISO 13629–2:2014 textiles—determination of antifungal activity of textile products—part 2: plate count method
International Organization for Standardization (2019) ISO 18184:2019 Textiles—Determination of antiviral activity of textile products
Japanese Industrial Standards (2020) JIS L 1903:2020 textiles-determination of antibacterial activity and efficacy of textile products
Jennifer MA (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48(5):16. https://doi.org/10.1093/JAC/48.SUPPL_1.5
Joshi M, Butola BS (2013) Application technologies for coating, lamination and finishing of technical textiles. Adv Dye Finish Tech Text. https://doi.org/10.1533/9780857097613.2.355
Kangwansupamonkon W, Lauruengtana V, Surassmo S, Ruktanonchai U (2009) Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomed Nanotechnol Biol Med 5:240–249. https://doi.org/10.1016/J.NANO.2008.09.004
Karagoz S, Burak Kiremitler N, Sarp G, Pekdemir S, Salem S, Goksu AG, Serdar Onses M, Sozdutmaz I, Sahmetlioglu E, Ozkara ES, Ceylan A, Yilmaz E (2021) Antibacterial, antiviral, and self-cleaning mats with sensing capabilities based on electrospun nanofibers decorated with ZnO nanorods and Ag nanoparticles for protective clothing applications. ACS Appl Mater Interfaces 13:5678–5690. https://doi.org/10.1021/acsami.0c15606
Karimi L, Yazdanshenas ME, Khajavi R, Rashidi A, Mirjalili M (2014) Using graphene/TiO2 nanocomposite as a new route for preparation of electroconductive, self-cleaning, antibacterial and antifungal cotton fabric without toxicity. Cellulose 21:3813–3827. https://doi.org/10.1007/S10570-014-0385-1
Kaweeteerawat C, Ubol N, Sangmuang S, Aueviriyavit S, Maniratanachote R (2017) Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J Toxicol Environ Health A 80:1276–1289. https://doi.org/10.1080/15287394.2017.1376727
Kelly FM, Johnston JH (2011) Colored and functional silver nanoparticle−wool fiber composites. ACS Appl Mater Interfaces 3:1083–1092. https://doi.org/10.1021/AM101224V
Krumm A (2020) Virus assays. In: BMG LABTECH. https://www.bmglabtech.com/virus-assays/. Accessed 16 Aug 2021
Kumar BS (2016) Study on antimicrobial effectiveness of sliver nano coating over cotton fabric through green approach. Int J Pharma Sci Res 7:363–368
Kumar S, Karmacharya M, Joshi SR, Gulenko O, Park J, Kim GH, Cho YK (2020) Photoactive antiviral face mask with self-sterilization and reusability. Nano Lett 21:337–343. https://doi.org/10.1021/acs.nanolett.0c03725
Lara HH, Ayala-Núñez NV, del Turrent LCI, Padilla CR (2010a) Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol 26:615–621. https://doi.org/10.1007/s11274-009-0211-3
Lara HH, Ayala-Núñez NV, del Turrent LCI, Padilla CR (2010b) Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology 8:1–10. https://doi.org/10.1186/1477-3155-8-1
Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK (2011) Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology 9:1–8. https://doi.org/10.1186/1477-3155-9-30
Latthe SS, Sutar RS, Kodag VS, Bhosale AK, Kumar AM, Kumar Sadasivuni K, Xing R, Liu S (2019) Self-cleaning superhydrophobic coatings: potential industrial applications. Prog Organ Coat 128:52–58. https://doi.org/10.1016/J.PORGCOAT.2018.12.008
Lau JY-N, Chan DSB, Chiou J, Kan CW, Lam WH, Yung KF (2018) Durable antimicrobial treatment of textile for use in healthcare environment
Li Y, Leung P, Yao L, Song QW, Newton E (2006) Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect 62:58–63. https://doi.org/10.1016/j.jhin.2005.04.015
Lin J, Zheng C, Ye WJ, Wang HQ, Feng DY, Li QY, Huan BW (2015) A facile dip-coating approach to prepare SiO2/fluoropolymer coating for superhydrophobic and superoleophobic fabrics with self-cleaning property. J Appl Polym Sci. https://doi.org/10.1002/APP.41458
Lord PR (2003) Textile products and fiber production. In: Handbook of yarn production, Woodhead Publishing, pp 18–55
Lumen Agglutination Assays. In: Lumen Microbiol. https://courses.lumenlearning.com/microbiology/chapter/agglutination-assays/. Accessed 12 Aug 2021
Malis D, Jeršek B, Tomšič B, Štular D, Golja B, Kapun G, Simončič B (2019) Antibacterial activity and biodegradation of cellulose fiber blends with incorporated ZnO. Materials 12:3399. https://doi.org/10.3390/ma12203399
Mazurkova NA, Spitsyna YE, Shikina NV et al (2010) Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnologies Russ 5:417–420. https://doi.org/10.1134/S1995078010050174
Mehrbod P, Motamed N, Tabatabaian M, Soleimani Estyar R, Amini E, Shahidi M, Kheiri MT (2009) In vitro antiviral effect of “nanosilver” on influenza virus. Daru 17:88–93
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346. https://doi.org/10.1088/0957-4484/16/10/059
Morris D, Ansar M, Speshock J, Ivanciuc T, Qu Y, Casola A, Garofalo R (2019) Antiviral and immunomodulatory activity of silver nanoparticles in experimental rsv infection. Viruses 11:723. https://doi.org/10.3390/v11080732
Naebe M, Haque ANMA, Haji A (2022) Plasma-assisted antimicrobial finishing of textiles: a review. Engineering 12:145–163. https://doi.org/10.1016/j.eng.2021.01.011
Naqvi AAT, Fatima K, Mohammad T, Fatima U, Singh IK, Singh A, Atif SM, Hariprasad G, Hasan GM, Hassan MdI (2020) Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. BBA Mol Basis Dis 1866:165878. https://doi.org/10.1016/j.bbadis.2020.165878
Noman MTT, Petrů M (2020) Functional properties of sonochemically synthesized zinc oxide nanoparticles and cotton composites. Nanomaterials 10:1661. https://doi.org/10.3390/NANO10091661
Onar N, Aksit AC, Sen Y, Mutlu M (2011) Antimicrobial, UV-protective and self-cleaning properties of cotton fabrics coated by dip-coating and solvothermal coating methods. Fibers Polym 12:461–470. https://doi.org/10.1007/S12221-011-0461-1
Pankaj K (2013) Virus identification and quantification. In: Labome. https://www.labome.com/method/Virus-Identification-and-Quantification.html. Accessed 12 Aug 2021
Pant HR, Bajgai MP, Nam KT, Seo YA, Pandeya DR, Hong ST, Kim HY (2011) Electrospun nylon-6 spider-net like nanofiber mat containing TiO2 nanoparticles: a multifunctional nanocomposite textile material. J Hazard Mater 185:124–130. https://doi.org/10.1016/J.JHAZMAT.2010.09.006
Perkas N, Perelshtein I, Gedanken A (2018) Coating textiles with antibacterial nanoparticles using the sonochemical techniqe. J Mach Constr Maint Probl Eksploat 4:15–26
Petkova P, Francesko A, Fernandes MM, Mendoza E, Perelshtein I, Gedanken A, Tzanov T (2014) Sonochemical coating of textiles with hybrid ZnO/chitosan antimicrobial nanoparticles. ACS Appl Mater Interfaces 6:1164–1172. https://doi.org/10.1021/am404852d|
Prasher P, Singh M, Mudila Harish (2018) Silver nanoparticles as antimicrobial therapeutics: current perspectives and future challenges. 3 Biotech 8:411. https://doi.org/10.1007/s13205-018-1436-3
Prorokova NP, Kumeeva TYu, Kuznetsov OYu (2018) Antimicrobial properties of polyester fabric modified by nanosized titanium dioxide. Inorg Mater Appl Res 9:250–256. https://doi.org/10.1134/S2075113318020235
Qin T, Ma R, Yin Y, Miao X, Chen S, Fan K, Xi J, Liu Q, Gu Y, Yin Y, Hu J, Liu X, Peng D, Gao L (2019) Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 9:6920–6935. https://doi.org/10.7150/thno.35826
Raghunath A, Perumal E (2017) Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int J Antimicrob Agents 49:137–152. https://doi.org/10.1016/j.ijantimicag.2016.11.011
Rai M, Deshmukh SD, Ingle AP, Gupta IR, Galdiero M, Galdiero S (2014) Metal nanoparticles: the protective nanoshield against virus infection. Crit Rev Microbiol 42:46–56. https://doi.org/10.3109/1040841X.2013.879849
Ranjbar-Mohammadi M, Ehsan Y (2021) Fabrication of a dye removal system through electrospun of TiO 2 /Nylon-6 nanocomposite on three-dimensional spacer fabrics. Polym Bull 79:2953–2967. https://doi.org/10.1007/s00289-021-03645-6
Rastgoo M, Montazer M, Malek RMA, Harifi T, Mahmoudi Rad M (2016) Ultrasound mediation for one-pot sonosynthesis and deposition of magnetite nanoparticles on cotton/polyester fabric as a novel magnetic, photocatalytic, sonocatalytic, antibacterial and antifungal textile. Ultrason Sonochem 31:257–266. https://doi.org/10.1016/J.ULTSONCH.2016.01.008
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4:707–716. https://doi.org/10.1016/J.ACTBIO.2007.11.006
Sahin F, Ustaoglu Z, Demirci S, Demir O, Asutay AB (2017) Antimicrobial and antiviral hygienic products
Salat M, Petkova P, Hoyo J, Perelshtein I, Gedanken A, Tzanov T (2018) Durable antimicrobial cotton textiles coated sonochemically with ZnO nanoparticles embedded in an in-situ enzymatically generated bioadhesive. Carbohydr Polym 189:198–203. https://doi.org/10.1016/J.CARBPOL.2018.02.033
Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N, Fauzi MB (2020) The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials. https://doi.org/10.3390/nano10081566
Sasaki K, Tenjimbayashi M, Manabe K, Shiratori S (2016) Asymmetric superhydrophobic/superhydrophilic cotton fabrics designed by spraying polymer and nanoparticles. ACS Appl Mater Interfaces 8:651–659. https://doi.org/10.1021/acsami.5b09782
Schindler WD, Hauser PJ (2004) Chemical finishing of textiles. Wood Head Publishing Ltd
Shahid M, Ali A, Khaleeq H, Farrukh Tahir M, Militky J, Wiener J (2021) Development of antimicrobial multifunctional textiles to avoid from hospital-acquired infections. Fibers Polym 22:3055–3067. https://doi.org/10.1007/s12221-021-0002-5
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581:221–224. https://doi.org/10.1038/s41586-020-2179-y
Sharma P, Pant S, Dave V, Tak K, Sadhu V, Reddy KR (2019) Green synthesis and characterization of copper nanoparticles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J Microbiol Methods 160:107–116. https://doi.org/10.1016/J.MIMET.2019.03.007
Shionoiri N, Sato T, Fujimori Y, Nakayama T, Nemoto M, Matsunaga T, Tanaka T (2012) Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J Biosci Bioeng 113:580–586. https://doi.org/10.1016/j.jbiosc.2011.12.006
Simončič B, Tomšič B (2017) Recent concepts of antimicrobial textile finishes. In: Mittal K, Bahners T (eds) Textile finishing, United States, pp 3–48
Sójka-Ledakowicz J, Chruściel JJ, Kudzin MH, Łatwińska M, Kiwała M (2016) Antimicrobial functionalization of textile materials with copper silicate. Fibres Text East Eur 24:151–156. https://doi.org/10.5604/12303666.1215541
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182. https://doi.org/10.1016/j.jcis.2004.02.012
Songer J (2020) Mask fabrics: introduction to fibers and fabrics. In: MakerMask. https://makermask.org/mask-fabrics-101-introduction-to-fibers-and-fabrics/. Accessed 14 Mar 2022
Swiss Standards (1994a) SN 195920:1994a textile fabrics—determination of the antibacterial activity—agar diffusion plate test
Swiss Standards (1994b) SN 195921:1994b textile fabrics—determination of the antimycotic activity—agar diffusion plate test
T for textile classification of textile fibers. http://gpktt.weebly.com/classification-of-textile-fibers.html. Accessed 23 Sep 2021
Tang X, Yan X (2017) Dip-coating for fibrous materials: mechanism, methods and applications. J Sol Gel Sci Technol 81:378–404. https://doi.org/10.1007/s10971-016-4197-7
Tania IS, Ali M (2021) Coating of ZnO nanoparticle on cotton fabric to create a functional textile with enhanced mechanical properties. Polymers 13:2701. https://doi.org/10.3390/POLYM13162701
Tavakoli A, Hashemzadeh MS (2020) Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J Virol Methods 275:113688. https://doi.org/10.1016/j.jviromet.2019.113688
Teufel L, Redl B (2006) Improved methods for the investigation of the interaction between textiles and microorganisms. Lenzing Berichte 85:1
Tijing LD, Ruelo MTG, Amarjargal A, Pant HR, Park CH, Kim DW, Kim CS (2012) Antibacterial and superhydrophilic electrospun polyurethane nanocomposite fibers containing tourmaline nanoparticles. Chem Eng J 197:41–48. https://doi.org/10.1016/J.CEJ.2012.05.005
Tomšič B, Simončič B, Orel B, Žerjav M, Schroers H, Simončič A, Samardžija Z (2009) Antimicrobial activity of AgCl embedded in a silica matrix on cotton fabric. Carbohydr Polym 75:618–626. https://doi.org/10.1016/J.CARBPOL.2008.09.013
Tourinho PS, van Gestel CAM, Lofts S, Svendsen C, Soares AMVM, Loureiro S (2012) Metal-based nanoparticles in soil: fate, behavior, and effects on soil invertebrates. Environ Toxicol Chem 31:1679–1692. https://doi.org/10.1002/ETC.1880
Tremiliosi G, Simoes LG, Minozzi D, Santos R, Vilela D, Durigon EL, Machado RRG, Medina DS, Ribeiro LK, Rosa ILV, Assis M, Andrés J, Longo E, Freitas-Junior L (2020) Ag nanoparticles-based antimicrobial polycotton fabrics to prevent the transmission and spread of SARS-CoV-2. BioRxiv. https://doi.org/10.1101/2020.06.26.152520
Tsuang Y-H, Sun J-S, Huang Y-C, Lu C-H, Chang WH-S, Wang C-C (2008) Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif Organs 32:167–174. https://doi.org/10.1111/J.1525-1594.2007.00530.X
Uddin F (2015) Encyclopedia of biomedical polymers and polymeric biomaterials. Taylor & Francis
Valério A, Sárria MP, Rodriguez-Lorenzo L, Hotza D, Espiña B, Gómez González SY (2020) Are TiO2 nanoparticles safe for photocatalysis in aqueous media? Nanoscale Adv 2:4951–4960. https://doi.org/10.1039/D0NA00584C
Vasantharaj S, Sathiyavimal S, Saravanan M, Senthilkumar P, Gnanasekaran K, Shanmugavel M, Manikandan E, Pugazhendhi A (2019) Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B 191:143–149. https://doi.org/10.1016/J.JPHOTOBIOL.2018.12.026
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181:281–292. https://doi.org/10.1016/j.cell.2020.02.058
Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J (2020a) Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181:894–904. https://doi.org/10.1016/j.cell.2020.03.045
Wang W, Li T, Liu K, Wang S, Peng H (2020b) Effects of three fabric weave textures on the electrochemical and electrical properties of reduced graphene/textile flexible electrodes. RSC Adv 10:6249–6258. https://doi.org/10.1039/C9RA08524F
Xu Q, Ke X, Ge N, Shen L, Zhang Y, Fu F, Liu X (2018) Preparation of copper nanoparticles coated cotton fabrics with durable antibacterial properties. Fibers Polym 19:1004–1013. https://doi.org/10.1007/S12221-018-8067-5
Yadav A, Prasad V, Kathe AA, Raj S, Yadav D, Sundaramoorthy C, Vigneshwaran N (2006) Functional finishing in cotton fabrics using zinc oxide nanoparticles. Bull Mater Sci 29:641–645. https://doi.org/10.1007/s12034-006-0017-y
Zhang F, Wu X, Chen Y, Lin H (2009) Application of silver nanoparticles to cotton fabric as an antibacterial textile finish. Fibers Polym 10:496–501. https://doi.org/10.1007/S12221-009-0496-8
Acknowledgments
Gabriela Zanchettin is a recipient of a CAPES scholarship. The authors are grateful to Brazilian agencies CAPES and CNPq for funding this work.
Funding
CAPES, CNPq, and Federal University of Santa Catarina.
Author information
Authors and Affiliations
Contributions
GZ: writing—original draft, conceptualization, investigation, visualization. SYGG and GSF: conceptualization, supervision, writing—review and editing, visualization. DH: supervision, writing—review and editing, funding acquisition, project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zanchettin, G., Falk, G.S., González, S.Y. et al. Tutorial review on the processing and performance of fabrics with antipathogenic inorganic agents. Cellulose 30, 2687–2712 (2023). https://doi.org/10.1007/s10570-023-05060-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05060-8