Abstract
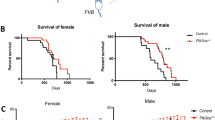
The transforming growth factor betas (TGFβs) are context-dependent regulators of neurons in vitro, but their physiological functions in the brain are unclear. Haploinsufficiency of either Tgfβ1 or Tgfβ2 leads to age-related deterioration of neurons, but the development of the brain is normal in the full absence of either of these genes. However, some individuals with mis-sense mutations of TGFβ receptors are mentally retarded, suggesting that the TGFβ isoforms can compensate for each other during brain development. This possibility was tested by generating mice (NSE × PTR) with neuron-specific expression of a dominant-negative inhibitor of TGFβ signaling. The NSE × PTR mice with a FVBxC57Bl/6 genetic background were viable and developed normally despite strong neuronal expression of the inhibitor of TGFβ signaling. Their cerebella were of normal size and contained normal numbers of neurons. When the genetic background of the mice was changed to C57BL/6, the phenotype of the mice became neonatal lethal, with the neonates exhibiting various malformations. The malformations correlated with sites of non-neuronal expression of the transgenes and included facial dysmorphogenesis, incomplete closure of the ventral body wall and absence of intestinal motility. The C57BL/6 Tgfbm1–3 alleles, which modulate the phenotype of Tgfβ1−/− mice, were not major determinants of the NSE × PTR phenotype. The data suggest that the development of the cerebellum is insensitive to the level of TGFβ signaling, although this may be dependent on the genetic background.






Similar content being viewed by others
References
Andrews ZB, Zhao H, Frugier T, Meguro R, Grattan DR, Koishi K, McLennan IS (2006) Transforming growth factor β2 haploinsufficient mice develop age-related nigrostriatal dopamine deficits. Neurobiol Dis 21(3):568–575. doi:10.1016/j.nbd.2005.09.001
Boivin GP, Molina JR, Ormsby I, Stemmermann G, Doetschman T (1996) Gastric lesions in transforming growth factor beta-1 heterozygous mice. Lab Invest 74(2):513–518
Bonyadi M, Rusholme SA, Cousins FM, Su HC, Biron CA, Farrall M, Akhurst RJ (1997) Mapping of a major genetic modifier of embryonic lethality in TGF beta 1 knockout mice. Nat Genet 15(2):207–211. doi:10.1038/ng0297-207
Brewer S, Williams T (2004) Finally, a sense of closure? Animal models of human ventral body wall defects. Bioessays 26(12):1307–1321. doi:10.1002/bies.20137
Brionne TC, Tesseur I, Masliah E, Wyss-Coray T (2003) Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40(6):1133–1145. doi:10.1016/S0896-6273(03)00766-9
Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ (1998) Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol 54(3):495–503
Day WA, Koishi K, McLennan IS (2003) Transforming growth factor beta 1 may regulate the stability of mature myelin sheaths. Exp Neurol 284(2):857–864. doi:10.1016/S0014-4886(03)00308-X
de Caestecker M (2004) The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev 15(1):1–11. doi:10.1016/j.cytogfr.2003.10.004
Dunker N, Krieglstein K (2002) TGFbeta2 (-/-) TGFbeta3 (-/-) double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol (Berl) 206(1–2):73–83. doi:10.1007/s00429-002-0273-6
Filvaroff EH, Ebner R, Derynck R (1994) Inhibition of myogenic differentiation in myoblasts expressing a truncated type II TGF-beta receptor. Development 120(5):1085–1095
Filvaroff E, Erlebacher A, Ye J, Gitelman SE, Lotz J, Heillman M, Derynck R (1999) Inhibition of TGF-beta receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development 126(19):4267–4279
Frugier T, Koishi K, Matthaei KI, McLennan IS (2005) Transgenic mice carrying a tetracycline-inducible, truncated transforming growth factor beta receptor (TbetaRII). Genesis 42(1):1–5. doi:10.1002/gene.20115
Gariepy CE (2004) Developmental disorders of the enteric nervous system: genetic and molecular bases. J Pediatr Gastroenterol Nutr 39(1):5–11. doi:10.1097/00005176-200407000-00003
Geiser AG, Zeng QQ, Sato M, Helvering LM, Hirano T, Turner CH (1998) Decreased bone mass and bone elasticity in mice lacking the transforming growth factor-beta1 gene. Bone 23(2):87–93. doi:10.1016/S8756-3282(98)00078-7
Glebova NO, Ginty DD (2005) Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28:191–222. doi:10.1146/annurev.neuro.28.061604.135659
Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ (1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96:857–881
Hashimoto M, Mikoshiba K (2004) Neuronal birthdate-specific gene transfer with adenoviral vectors. J Neurosci 24(1):286–296. doi:10.1523/JNEUROSCI.2529-03.2004
Honjo Y, Bian Y, Kawakami K, Molinolo A, Longenecker G, Boppana R, Larsson J, Karlsson S, Gutkind JS, Puri RK, Kulkarni AB (2007a) TGF-beta receptor I conditional knockout mice develop spontaneous squamous cell carcinoma. Cell Cycle 6(11):1360–1366
Honjo Y, Nagineni CN, Larsson J, Nandula SR, Hooks JJ, Chan CC, Karlsson S, Kulkarni AB (2007b) Neuron-specific TGF-beta signaling deficiency results in retinal detachment and cataracts in mice. Biochem Biophys Res Commun 352(2):418–422. doi:10.1016/j.bbrc.2006.11.033
Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J (1995) Abnormal lung development and cleft palate in mice lacking TGF-beta3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11:415–421. doi:10.1038/ng1295-415
Krieglstein K, Reuss B, Maysinger D, Unsicker K (1998) Transforming growth factor-beta mediates the neurotrophic effect of fibroblast growth factor-2 on midbrain dopaminergic neurons. Eur J Neurosci 10:2746–2750. doi:10.1046/j.1460-9568.1998.00324.x
Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S (1993) Transforming growth factor-beta1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 90(2):770–774. doi:10.1073/pnas.90.2.770
Kusakabe M, Cheong PL, Nikfar R, McLennan IS, Koishi K (2008) The structure of the TGF-beta latency associated peptide region determines the ability of the proprotein convertase furin to cleave TGF-betas. J Cell Biochem 103(1):311–320. doi:10.1002/jcb.21407
Letterio JJ, Bottinger EP (1998) TGF-beta knockout and dominant-negative receptor transgenic mice. Miner Electrolyte Metab 24(2–3):161–167. doi:10.1159/000057365
Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC (2005) A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37(3):275–281. doi:10.1038/ng1511
Madalosso SH, Perez-Villegas EM, Armengol JA (2005) Naturally occurring neuronal death during the postnatal development of Purkinje cells and their precerebellar afferent projections. Brain Res Brain Res Rev 49(2):267–279. doi:10.1016/j.brainresrev.2004.10.001
McLennan IS, Koishi K (2002) The transforming growth factor-betas: multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol 46(4):559–567
McLennan IS, Koishi K (2004) Fetal and maternal transforming growth factor-beta1 may combine to maintain pregnancy in mice. Biol Reprod 70(6):1614–1618. doi:10.1095/biolreprod.103.026179
McLennan IS, Taylor-Jeffs J (2004) The use of sodium lamps to brightly illuminate mouse houses during their dark phases. Lab Anim 38(4):1–9. doi:10.1258/0023677041958927
Millan FA, Denhez F, Kondaiah P, Akhurst RJ (1991) Embryonic gene expression patterns of TGF beta-1, beta-2 and beta-3 suggest different developmental functions in vivo. Development 111:131–144
Oshima M, Oshima H, Taketo MM (1996) TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179(1):297–302. doi:10.1006/dbio.1996.0259
Russell FD, Koishi K, Jiang Y, McLennan IS (2000) Anterograde axonal transport of glial cell line-derived neurotrophic factor and its receptors in rat hypoglossal nerve. Neuroscience 97(3):575–580. doi:10.1016/S0306-4522(00)00079-8
Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T (1997) TGF-beta2 knockout mice have multiple developmental defects that are non-overlapping with other TGF-beta knockout phenotypes. Development 124(13):2659–2670
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113(6):685–700. doi:10.1016/S0092-8674(03)00432-X
Tang Y, McKinnon ML, Leong LM, Rusholme AB, Wang S, Akhurst RJ (2003) Genetic modifiers interact with maternal determinants in vascular development of TGFbeta1-/- mice. Hum Mol Genet 12:1579–1589. doi:10.1093/hmg/ddg164
Tang Y, Sook Lee K, Yang H, Logan DW, Wang S, McKinnon ML, Holt LJ, Condie A, Luu MT, Akhurst RJ (2005) Epistatic interactions between modifier genes confer strain-specific redundancy for Tgfb1 in developmental angiogenesis. Genomics 85(1):60–70. doi:10.1016/j.ygeno.2004.09.003
Wang PY, Koishi K, McGeachie AB, Kimber M, Maclaughlin DT, Donahoe PK, McLennan IS (2005) Mullerian inhibiting substance acts as a motor neuron survival factor in vitro. Proc Natl Acad Sci USA 102(45):16421–16425. doi:10.1073/pnas.0508304102
Wang PY, Koishi K, McLennan IS (2007) BMP6 is axonally transported by motoneurons and supports their survival in vitro. Mol Cell Neurosci 34(4):653–661. doi:10.1016/j.mcn.2007.01.008
Acknowledgments
We thank Mrs. Nichola Batchelor for her expert care of the murine colonies and Mrs. Janet McLay for her technical support. The support of the Marsden Fund (Royal Society of New Zealand) is acknowledged, with our appreciation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asano, Y., Koishi, K., Frugier, T. et al. Mice with Disrupted TGFβ Signaling Have Normal Cerebella Development, but Exhibit Facial Dysmorphogenesis and Strain-Dependent Deficits in Their Body Wall. Cell Mol Neurobiol 29, 621–633 (2009). https://doi.org/10.1007/s10571-009-9354-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9354-x