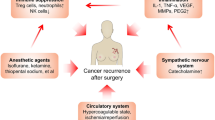
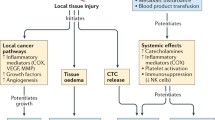
Abstract
Surgery remains the curative treatment modality for colorectal cancer in all stages, including stage IV with resectable liver metastasis. There is emerging evidence that the stress response caused by surgery as well as other perioperative therapies such as anesthesia and analgesia may promote growth of pre-existing micro-metastasis or potentially initiate tumor dissemination. Therapeutically targeting the perioperative period may therefore reduce the effect that surgical treatments have in promoting metastases, for example by combining β-adrenergic receptor antagonists and cyclooxygenase-2 (COX-2) inhibitors in the perioperative setting. In this paper, we highlight some of the mechanisms that may underlie surgery-related metastatic development in colorectal cancer. These include direct tumor spillage at the time of surgery, suppression of the anti-tumor immune response, direct stimulatory effects on tumor cells, and activation of the coagulation system. We summarize in more detail results that support a role for catecholamines as major drivers of the pro-metastatic effect induced by the surgical stress response, predominantly through activation of β-adrenergic signaling. Additionally, we argue that an improved understanding of surgical stress-induced dissemination, and more specifically whether it impacts on the level and nature of heterogeneity within residual tumor cells, would contribute to the successful clinical targeting of this process. Finally, we provide a proof-of-concept demonstration that ex-vivo analyses of colorectal cancer patient-derived samples using RGB-labeling technology can provide important insights into the heterogeneous sensitivity of tumor cells to stress signals. This suggests that intra-tumor heterogeneity is likely to influence the efficacy of perioperative β-adrenergic receptor and COX-2 inhibition, and that ex-vivo characterization of heterogeneous stress response in tumor samples can synergize with other models to optimize perioperative treatments and further improve outcome in colorectal and other solid cancers.



Similar content being viewed by others
Abbreviations
- COX-2:
-
Cyclooxygenase 2
- β-AR:
-
Beta-adrenergic receptor
- CRP:
-
C-reactive protein
- Tregs:
-
Regulatory T-cells
- CTCs:
-
Circulating tumor cells
- ctDNA:
-
Circulating tumor DNA
- VEGF:
-
Vascular endothelial growth factor
- MMP:
-
Matrix metallo-proteinase
- DPC:
-
Digital phase contrast
References
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372(14):1324–1332. https://doi.org/10.1056/NEJMoa1414882
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70(14):5649–5669. https://doi.org/10.1158/0008-5472.CAN-10-1040
Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S (2015) Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 12(4):213–226. https://doi.org/10.1038/nrclinonc.2014.224
Nicoud IB, Jones CM, Pierce JM, Earl TM, Matrisian LM, Chari RS, Gorden DL (2007) Warm hepatic ischemia-reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase-9 induction. Cancer Res 67(6):2720–2728. https://doi.org/10.1158/0008-5472.can-06-3923
Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA (2014) A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother 63(11):1115–1128. https://doi.org/10.1007/s00262-014-1617-9
Desborough JP (2000) The stress response to trauma and surgery. Br J Anaesth 85(1):109–117
Tai LH, de Souza CT, Belanger S, Ly L, Alkayyal AA, Zhang J, Rintoul JL, Ananth AA, Lam T, Breitbach CJ, Falls TJ, Kirn DH, Bell JC, Makrigiannis AP, Auer RA (2013) Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res 73(1):97–107. https://doi.org/10.1158/0008-5472.can-12-1993
Kang KM, Hong KS, Noh GT, Oh B-Y, Chung SS, Lee R-A, Kim KH (2013) Optimal time of initiating adjuvant chemotherapy after curative surgery in colorectal cancer patients. Ann Coloproctol 29(4):150–154. https://doi.org/10.3393/ac.2013.29.4.150
Kennedy JM, Riji AM (1998) Effects of surgery on the pharmacokinetic parameters of drugs. Clin Pharmacokinet 35(4):293–312
Nepomniashchikh VA, Lomivorotov VV, Deryagin MN, Kniazkova LG, Novikov MA (2006) Surgical stress and its impact on hepatic metabolism and lipid peroxidation in cardiac patients: P-119. Eur J Anaesthesiol 23:40–41
Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, Arevalo JMG, Ma J, Horowitz M, Cole SW, Ben-Eliyahu S (2017) Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. https://doi.org/10.1158/1078-0432.ccr-17-0152
Ceelen WP, Bracke ME Peritoneal minimal residual disease in colorectal cancer: mechanisms, prevention, and treatment. Lancet Oncol 10 (1):72–79. https://doi.org/10.1016/S1470-2045(08)70335-8
Lim SH, Spring KJ, de Souza P, MacKenzie S, Bokey L (2015) Circulating tumour cells and circulating nucleic acids as a measure of tumour dissemination in non-metastatic colorectal cancer surgery. Eur J Surg Oncol 41(3):309–314. https://doi.org/10.1016/j.ejso.2014.12.005
Guo N, Lou F, Ma Y, Li J, Yang B, Chen W, Ye H, Zhang J-B, Zhao M-Y, Wu W-J, Shi R, Jones L, Chen KS, Huang XF, Chen S-Y, Liu Y (2016) Circulating tumor DNA detection in lung cancer patients before and after surgery. 6:33519. https://doi.org/10.1038/srep33519. https://www.nature.com/articles/srep33519
Lynch ML, Brand MI (2005) Preoperative evaluation and oncologic principles of colon cancer surgery. Clin Colon Rectal Surg 18(3):163–173. https://doi.org/10.1055/s-2005-916277
(NCCN). NCCN (2016) Rectal cancer guidelines. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.. Accessed 17 Mar 2016
Binda MM, Corona R, Amant F, Koninckx PR (2014) Conditioning of the abdominal cavity reduces tumor implantation in a laparoscopic mouse model. Surg Today 44(7):1328–1335. https://doi.org/10.1007/s00595-014-0832-5
Takemoto K, Shiozaki A, Ichikawa D, Komatsu S, Konishi H, Nako Y, Murayama Y, Kuriu Y, Nakanishi M, Fujiwara H, Okamoto K, Sakakura C, Nakahari T, Marunaka Y, Otuji E (2015) Evaluation of the efficacy of peritoneal lavage with distilled water in colorectal cancer surgery: in vitro and in vivo study. J Gastroenterol 50(3):287–297. https://doi.org/10.1007/s00535-014-0971-x
Pattana-arun J, Wolff BG (2008) Benefits of povidone-iodine solution in colorectal operations: science or legend. Dis Colon Rectum 51(6):966–971. https://doi.org/10.1007/s10350-008-9213-8
Alkhamesi NA, Ziprin P, Pfistermuller K, Peck DH, Darzi AW (2005) ICAM-1 mediated peritoneal carcinomatosis, a target for therapeutic intervention. Clin Exp Metastasis 22(6):449–459. https://doi.org/10.1007/s10585-005-2893-8
Andersson B, Ansari D, Nordén M, Nilsson J, Andersson R (2013) Surgical stress response after colorectal resection. Int Surg 98(4):292–299. https://doi.org/10.9738/INTSURG-D-12-00009.1
Brokelman WJA, Lensvelt M, Rinkes IHMB., Klinkenbijl JHG, Reijnen MMPJ. (2011) Peritoneal changes due to laparoscopic surgery. Surg Endosc 25(1):1–9. https://doi.org/10.1007/s00464-010-1139-2
Jessy T (2011) Immunity over inability: the spontaneous regression of cancer. J Nat Sci Biol Med 2(1):43–49. https://doi.org/10.4103/0976-9668.82318
Lippey J, Bousounis R, Behrenbruch C, McKay B, Spillane J, Henderson MA, Speakman D, Gyorki DE (2016) Intralesional PV-10 for in-transit melanoma-a single-center experience. J Surg Oncol 114(3):380–384. https://doi.org/10.1002/jso.24311
Siekmann W, Eintrei C, Magnuson A, Sjolander A, Matthiessen P, Myrelid P, Gupta A (2017) Surgical and not analgesic technique affects postoperative Inflammation following colorectal cancer surgery: a prospective, randomized study. Colorectal Dis. https://doi.org/10.1111/codi.13643
Zawadzki M, Krzystek-Korpacka M, Gamian A, Witkiewicz W (2017) Comparison of inflammatory responses following robotic and open colorectal surgery: a prospective study. Int J Colorectal Dis 32(3):399–407. https://doi.org/10.1007/s00384-016-2697-0
Shibata J, Ishihara S, Tada N, Kawai K, Tsuno NH, Yamaguchi H, Sunami E, Kitayama J, Watanabe T (2015) Surgical stress response after colorectal resection: a comparison of robotic, laparoscopic, and open surgery. Tech Coloproctol 19(5):275–280. https://doi.org/10.1007/s10151-014-1263-4
Whelan RL, Franklin M, Holubar SD, Donahue J, Fowler R, Munger C, Doorman J, Balli JE, Glass J, Gonzalez JJ, Bessler M, Xie H, Treat M (2003) Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc 17(6):972–978. https://doi.org/10.1007/s00464-001-8263-y
Ferri M, Rossi Del Monte S, Salerno G, Bocchetti T, Angeletti S, Malisan F, Cardelli P, Ziparo V, Torrisi MR, Visco V (2013) Recovery of immunological homeostasis positively correlates both with early stages of right-colorectal cancer and laparoscopic surgery. PLoS ONE 8(9):e74455. https://doi.org/10.1371/journal.pone.0074455
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82. https://doi.org/10.1002/bjs.8945
Wu FP, Sietses C, von Blomberg BM, van Leeuwen PA, Meijer S, Cuesta MA (2003) Systemic and peritoneal inflammatory response after laparoscopic or conventional colon resection in cancer patients: a prospective, randomized trial. Dis Colon Rectum 46(2):147–155. https://doi.org/10.1097/01.dcr.0000049321.18644.08
Hill AG, Connolly AB (2006) Minimal access colonic surgery: is it truly minimally invasive? ANZ J Surg 76(5):282–284. https://doi.org/10.1111/j.1445-2197.2006.03711.x
Jie H-Y, Ye J-L, Zhou H-H, Li Y-X (2014) Perioperative restricted fluid therapy preserves immunological function in patients with colorectal cancer. World J Gastroenterol 20(42):15852–15859. https://doi.org/10.3748/wjg.v20.i42.15852
Mlecnik B, Bindea G, Kirilovsky A, Angell HK, Obenauf AC, Tosolini M, Church SE, Maby P, Vasaturo A, Angelova M, Fredriksen T, Mauger S, Waldner M, Berger A, Speicher MR, Pages F, Valge-Archer V, Galon J (2016) The tumor microenvironment and immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med 8(327):327ra326. https://doi.org/10.1126/scitranslmed.aad6352
Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG (2000) Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer 82(5):1009–1012. https://doi.org/10.1054/bjoc.1999.1034
Neeman E, Zmora O, Ben-Eliyahu S (2012) A new approach to reducing post-surgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res 18(18):4895–4902. https://doi.org/10.1158/1078-0432.CCR-12-1087
Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S (2003) Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol 10(4):469–479
Ramirez MF, Ai D, Bauer M, Vauthey JN, Gottumukkala V, Kee S, Shon D, Truty M, Kuerer HM, Kurz A, Hernandez M, Cata JP (2015) Innate immune function after breast, lung, and colorectal cancer surgery. J Surg Res 194(1):185–193. https://doi.org/10.1016/j.jss.2014.10.030
Donadon M, Hudspeth K, Cimino M, Di Tommaso L, Preti M, Tentorio P, Roncalli M, Mavilio D, Torzilli G (2017) Increased infiltration of natural killer and T cells in colorectal liver metastases improves patient overall survival. J Gastrointest Surg. https://doi.org/10.1007/s11605-017-3446-6
Pugh SA, Harrison RJ, Primrose JN, Khakoo SI (2014) T cells but not NK cells are associated with a favourable outcome for resected colorectal liver metastases. BMC Cancer 14:180. https://doi.org/10.1186/1471-2407-14-180
Brackett CM, Kojouharov B, Veith J, Greene KF, Burdelya LG, Gollnick SO, Abrams SI, Gudkov AV (2016) Toll-like receptor-5 agonist, entolimod, suppresses metastasis and induces immunity by stimulating an NK-dendritic-CD8 + T-cell axis. Proc Natl Acad Sci USA 113(7):E874–E883. https://doi.org/10.1073/pnas.1521359113
Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton V, Colpitts SL, Beauchemin N, Saleh M (2015) The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity 43(4):751–763. https://doi.org/10.1016/j.immuni.2015.08.013
Kee JY, Ito A, Hojo S, Hashimoto I, Igarashi Y, Tsukada K, Irimura T, Shibahara N, Nakayama T, Yoshie O, Sakurai H, Saiki I, Koizumi K (2013) Chemokine CXCL16 suppresses liver metastasis of colorectal cancer via augmentation of tumor-infiltrating natural killer T cells in a murine model. Oncol Rep 29(3):975–982. https://doi.org/10.3892/or.2012.2185
Weese JL, Emoto SE, Sondel PM (1987) Reduced incidence of hepatic metastases by perioperative treatment with recombinant human interleukin-2. Dis Colon Rectum 30(7):503–507
Shakhar G, Ben-Eliyahu S (2003) Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol 10(8):972–992
Nichols PH, Ramsden CW, Ward U, Sedman PC, Primrose JN (1992) Perioperative immunotherapy with recombinant interleukin 2 in patients undergoing surgery for colorectal cancer. Cancer Res 52(20):5765–5769
Brivio F, Fumagalli L, Chiarelli M, Denova M, Bertolini A, Cetta M, Nespoli A (2007) [Immunotherapy in radical surgery of colorectal carcinoma]. Chir Ital 59(5):635–640
Tai LH, Zhang J, Auer RC (2013) Preventing surgery-induced NK cell dysfunction and cancer metastases with influenza vaccination. Oncoimmunology 2(11):e26618. https://doi.org/10.4161/onci.26618
Neeman E, Ben-Eliyahu S (2013) The perioperative period and promotion of cancer metastasis: new outlooks on mediating mechanisms and immune involvement. Brain Behav Immun 30(Suppl):S32–S40. https://doi.org/10.1016/j.bbi.2012.03.006
Sorski L, Melamed R, Matzner P, Lavon H, Shaashua L, Rosenne E, Ben-Eliyahu S (2016) Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun 58:91–98. https://doi.org/10.1016/j.bbi.2016.05.017
Coelho M, Soares-Silva C, Brandao D, Marino F, Cosentino M, Ribeiro L (2017) Beta-adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol 143(2):275–291. https://doi.org/10.1007/s00432-016-2278-1
Masur K, Niggemann B, Zanker KS, Entschladen F (2001) Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 61(7):2866–2869
Chin CC, Li JM, Lee KF, Huang YC, Wang KC, Lai HC, Cheng CC, Kuo YH, Shi CS (2016) Selective beta2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J Cell Physiol 231(2):459–472. https://doi.org/10.1002/jcp.25092
Barron TI, Sharp L, Visvanathan K (2012) Beta-adrenergic blocking drugs in breast cancer: a perspective review. Ther Adv Med Oncol 4(3):113–125. https://doi.org/10.1177/1758834012439738
Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S (2008) Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. https://doi.org/10.1245/s10434-10008-19890-10435
Caine GJ, Stonelake PS, Lip GY, Kehoe ST (2002) The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 4(6):465–473. https://doi.org/10.1038/sj.neo.7900263
Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC (1989) Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett 242(2):237–239
Levi M, Keller TT, van Gorp E, ten Cate H (2003) Infection and inflammation and the coagulation system. Cardiovasc Res 60(1):26–39
Bleeker JS, Hogan WJ (2011) Thrombocytosis: diagnostic evaluation, thrombotic risk stratification, and risk-based management strategies. Thrombosis 2011:536062. https://doi.org/10.1155/2011/536062
Seth R, Tai LH, Falls T, de Souza CT, Bell JC, Carrier M, Atkins H, Boushey R, Auer RA (2013) Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann Surg 258(1):158–168. https://doi.org/10.1097/SLA.0b013e31826fcbdb
Gay LJ, Felding-Habermann B (2011) Contribution of platelets to tumour metastasis. Nat Rev Cancer 11(2):123–134
Coyle C, Cafferty FH, Rowley S, MacKenzie M, Berkman L, Gupta S, Pramesh CS, Gilbert D, Kynaston H, Cameron D, Wilson RH, Ring A, Langley RE, Add-aspirin i (2016) ADD-ASPIRIN: a phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp Clin Trials 51:56–64. https://doi.org/10.1016/j.cct.2016.10.004
Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M (2010) Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg 110(6):1636–1643. https://doi.org/10.1213/ANE.0b013e3181de0ab6
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S (2003) Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg 97(5):1331–1339
Ahlers O, Nachtigall I, Lenze J, Goldmann A, Schulte E, Hohne C, Fritz G, Keh D (2008) Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth 101(6):781–787. https://doi.org/10.1093/bja/aen287
Heaney A, Buggy DJ (2012) Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth 109(Suppl 1):i17–i.28. https://doi.org/10.1093/bja/aes421
Patel S, Lutz JM, Panchagnula U, Bansal S (2012) Anesthesia and perioperative management of colorectal surgical patients—a clinical review (Part 1). J Anaesthesiol Clin Pharmacol 28(2):162–171. https://doi.org/10.4103/0970-9185.94831
Das J, Kumar S, Khanna S, Mehta Y (2014) Are we causing the recurrence-impact of perioperative period on long-term cancer prognosis: review of current evidence and practice. J Anaesthesiol Clin Pharmacol 30(2):153–159. https://doi.org/10.4103/0970-9185.129996
Atzil S, Arad M, Glasner A, Abiri N, Avraham R, Greenfeld K, Rosenne E, Beilin B, Ben-Eliyahu S (2008) Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology 109(6):989–997. https://doi.org/10.1097/ALN.0b013e31818ddb72
Sajid MS, Mallick AS, Rimpel J, Bokari SA, Cheek E, Baig MK (2008) Effect of heated and humidified carbon dioxide on patients after laparoscopic procedures: a meta-analysis. Surg Laparosc Endosc Percutaneous Tech 18(6):539–546. https://doi.org/10.1097/SLE.0b013e3181886ff4
Mari G, Crippa J, Costanzi A, Mazzola M, Rossi M, Maggioni D (2016) ERAS protocol reduces IL-6 secretion in colorectal laparoscopic surgery: results from a randomized clinical trial. Surg Laparosc Endosc Percutaneous Tech 26(6):444–448. https://doi.org/10.1097/sle.0000000000000324
Mari G, Costanzi A, Crippa J, Falbo R, Miranda A, Rossi M, Berardi V, Maggioni D (2016) Surgical stress reduction in elderly patients undergoing elective colorectal laparoscopic surgery within an ERAS protocol. Chirurgia 111(6):476–480. https://doi.org/10.21614/chirurgia.111.6.476
Lee J-W, Shahzad MMK, Lin YG, Armaiz-Pena G, Han H-D, Kim H-S, Nam EJ, Jennings NB, Halder J, Mangala LS, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK (2009) Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res 15(8):2695–2702. https://doi.org/10.1158/1078-0432.CCR-08-2966
Tai L-H, Tanese de Souza C, Sahi S, Zhang J, Alkayyal AA, Ananth AA, Auer RAC (2014) A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J Vis Exp (85):51253. https://doi.org/10.3791/51253
Choy C, Raytis JL, Smith DD, Duenas M, Neman J, Jandial R, Lew MW (2016) Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: the potential benefit of perioperative beta-blockade. Oncol Rep 35(6):3135–3142. https://doi.org/10.3892/or.2016.4710
Pisco AO, Huang S (2015) Non-genetic cancer cell plasticity and therapy-induced stemness in tumour relapse: ‘what does not kill me strengthens me’. Br J Cancer 112:1725. https://doi.org/10.1038/bjc.2015.146
Almendro V, Kim HJ, Cheng YK, Gonen M, Itzkovitz S, Argani P, van Oudenaarden A, Sukumar S, Michor F, Polyak K (2014) Genetic and phenotypic diversity in breast tumor metastases. Cancer Res 74(5):1338–1348. https://doi.org/10.1158/0008-5472.can-13-2357-t
Meacham CE, Morrison SJ (2013) Tumour heterogeneity and cancer cell plasticity. Nature 501(7467):328–337. https://doi.org/10.1038/nature12624
Kreso A, Dick JE (2014) Evolution of the cancer stem cell model. Cell Stem Cell 14(3):275–291. https://doi.org/10.1016/j.stem.2014.02.006
Kim K-T, Lee HW, Lee H-O, Kim SC, Seo YJ, Chung W, Eum HH, Nam D-H, Kim J, Joo KM, Park W-Y (2015) Single-cell mRNA sequencing identifies subclonal heterogeneity in anti-cancer drug responses of lung adenocarcinoma cells. Genome Biol 16(1):127. https://doi.org/10.1186/s13059-015-0692-3
Weber K, Thomaschewski M, Benten D, Fehse B (2012) RGB marking with lentiviral vectors for multicolor clonal cell tracking. Nat Protoc 5(4):839–849
Grillet F, Bayet E, Villeronce O, Zappia L, Lagerqvist EL, Lunke S, Charafe-Jauffret E, Pham K, Molck C, Rolland N, Bourgaux JF, Prudhomme M, Philippe C, Bravo S, Boyer JC, Canterel-Thouennon L, Taylor GR, Hsu A, Pascussi JM, Hollande F, Pannequin J (2016) Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut. https://doi.org/10.1136/gutjnl-2016-311447
Kakumoto M, Sakaeda T, Takara K, Nakamura T, Kita T, Yagami T, Kobayashi H, Okamura N, Okumura K (2003) Effects of carvedilol on MDR1-mediated multidrug resistance: comparison with verapamil. Cancer Sci 94(1):81–86
Bachmakov I, Werner U, Endress B, Auge D, Fromm MF (2006) Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol 20(3):273–282. https://doi.org/10.1111/j.1472-8206.2006.00408.x
Yang WL, Frucht H (2000) Cholinergic receptor up-regulates COX-2 expression and prostaglandin E(2) production in colon cancer cells. Carcinogenesis 21(10):1789–1793
Li M, Tan SY, Wang XF (2014) Paeonol exerts an anticancer effect on human colorectal cancer cells through inhibition of PGE(2) synthesis and COX-2 expression. Oncol Rep 32(6):2845–2853. https://doi.org/10.3892/or.2014.3543
Cervantes-Madrid DL, Nagi S, Asting Gustafsson A (2017) FosB transcription factor regulates COX-2 expression in colorectal cancer cells without affecting PGE2 expression. Oncol Lett 13(3):1411–1416. https://doi.org/10.3892/ol.2017.5571
Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID (2008) The effects of surgery on tumor growth: a century of investigations. Ann Oncol 19(11):1821–1828. https://doi.org/10.1093/annonc/mdn386
Coelho M, Moz M, Correia G, Teixeira A, Medeiros R, Ribeiro L (2015) Antiproliferative effects of beta-blockers on human colorectal cancer cells. Oncol Rep 33(5):2513–2520. https://doi.org/10.3892/or.2015.3874
Wong HP, Ho JW, Koo MW, Yu L, Wu WK, Lam EK, Tai EK, Ko JK, Shin VY, Chu KM, Cho CH (2011) Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sci 88(25–26):1108–1112. https://doi.org/10.1016/j.lfs.2011.04.007
Lin Q, Wang F, Yang R, Zheng X, Gao H, Zhang P (2013) Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS ONE 8(4):e61435. https://doi.org/10.1371/journal.pone.0061435
Acknowledgements
The authors thank Dr. Erica Sloan for supplying the pharmacological compounds and acknowledge the financial support of CSSANZ and Covidien. They also wish to thank the University of Melbourne BOMP facility for their support with imaging experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest in relation with this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Behrenbruch, C., Shembrey, C., Paquet-Fifield, S. et al. Surgical stress response and promotion of metastasis in colorectal cancer: a complex and heterogeneous process. Clin Exp Metastasis 35, 333–345 (2018). https://doi.org/10.1007/s10585-018-9873-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-018-9873-2