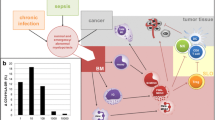
Abstract
Granulocyte-colony stimulating factor (G-CSF) is one of several cytokines that can expand and mobilize haematopoietic precursor cells from bone marrow. In particular, G-CSF mobilizes neutrophils when the host is challenged by infection or tissue damage. Severe neutropenia, or febrile neutropenia is a life-threatening event that can be mitigated by administration of G-CSF. Consequently, G-CSF has been used to support patients undergoing chemotherapy who would otherwise require dose reduction due to neutropenia. Over the past 10–15 years it has become increasingly apparent, in preclinical tumour growth and metastasis models, that G-CSF can support tumour progression by mobilization of tumour-associated neutrophils which consequently promote tumour dissemination and metastasis. With the increasing use of G-CSF in the clinic, it is pertinent to ask if there is any evidence of a similar promotion of tumour progression in patients. Here, we have reviewed the preclinical and clinical data on the potential contribution of G-CSF to tumour progression. We conclude that, whilst the evidence for a promotion of metastasis is strong in preclinical models and that limited data indicate that high serum G-CSF levels in patients are associated with poorer prognosis, no studies published so far have revealed evidence of increased tumour progression associated with supportive G-CSF use during chemotherapy in patients. Analysis of G-CSF receptor positive cohorts within supportive trials, as well as studies of the role of G-CSF blockade in appropriate tumours in the absence of chemotherapy could yield clinically translatable findings.

Similar content being viewed by others
Abbreviations
- G-CSF:
-
Granulocyte-colony stimulating factor
- CSF-1:
-
Colony stimulating factor-1
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor
- DFS:
-
Disease-free survival
- PFS:
-
Progression-free survival
- RFS:
-
Recurrence-free survival
- BCSS:
-
Breast cancer specific survival
- OS:
-
Overall survival
- MDSC:
-
Myeloid derived suppressor cells
- NET:
-
Neutrophil extracellular trap
- NLR:
-
Neutrophil to lymphocyte ratio
- CIN:
-
Chemotherapy induced neutropenia
- RDI:
-
Relative dose intensity
- RR:
-
Rate ratio
- HR:
-
Hazard ratio
References
Burgess AW, Metcalf D (1980) The nature and action of granulocyte-macrophage colony stimulating factors. Blood 56(6):947–958
Alexander WS (2016) In vivo at last: demonstrating the biological credentials and clinical potential of GM-CSF. Exp Hematol 44(8):669–673
Lopez AF, Nicola NA, Burgess AW, Metcalf D, Battye FL, Sewell WA et al (1983) Activation of granulocyte cytotoxic function by purified mouse colony-stimulating factors. J Immunol 131(6):2983–2988
Fujisawa M, Kobayashi Y, Okabe T, Takaku F, Komatsu Y, Itoh S (1986) Recombinant human granulocyte colony-stimulating factor induces granulocytosis in vivo. Jpn J Cancer Res 77(9):866–869
Lyman GH, Reiner M, Morrow PK, Crawford J (2015) The effect of filgrastim or pegfilgrastim on survival outcomes of patients with cancer receiving myelosuppressive chemotherapy. Ann Oncol 26(7):1452–1458
Barreda DR, Hanington PC, Belosevic M (2004) Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol 28(5):509–554
Hamilton JA, Cook AD, Tak PP (2017) Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat Rev Drug Discov 16(1):53–70
Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF et al (2008) A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood 112(13):5193–5201
Basu S, Hodgson G, Katz M, Dunn AR (2002) Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 100(3):854–861
Panopoulos AD, Watowich SS (2008) Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42(3):277–288
Joshi A, Pooley C, Freeman TC, Lennartsson A, Babina M, Schmidl C et al (2015) Technical advance: transcription factor, promoter, and enhancer utilization in human myeloid cells. J Leukoc Biol 97(5):985–995
Gerharz CD, Reinecke P, Schneider EM, Schmitz M, Gabbert HE (2001) Secretion of GM-CSF and M-CSF by human renal cell carcinomas of different histologic types. Urology 58(5):821–827
Steube KG, Meyer C, Tachibana M, Murai M, Drexler HG (1998) Bladder carcinoma cell line KU-19-19-derived cytokines support proliferation of growth factor-dependent hematopoietic cell lines: modulation by phorbol ester, interferon-gamma and interleukin-1 beta. Biochem Biophys Res Commun 242(3):497–501
Tachibana M, Miyakawa A, Nakashima J, Murai M, Nakamura K, Kubo A et al (1997) Constitutive production of multiple cytokines and a human chorionic gonadotrophin beta-subunit by a human bladder cancer cell line (KU-19-19): possible demonstration of totipotential differentiation. Br J Cancer 76(2):163–174
Steube KG, Meyer C, Drexler HG (1998) Secretion of functional hematopoietic growth factors by human carcinoma cell lines. Int J Cancer 78(1):120–124
Nitta T, Sato K, Allegretta M, Brocke S, Lim M, Mitchell DJ et al (1992) Expression of granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor genes in human astrocytoma cell lines and in glioma specimens. Brain Res 571(1):19–25
Kikuchi T, Nakahara S, Abe T (1996) Granulocyte colony-stimulating factor (G-CSF) production by astrocytoma cells and its effect on tumor growth. J Neurooncol 27(1):31–38
Morales-Arias J, Meyers PA, Bolontrade MF, Rodriguez N, Zhou Z, Reddy K et al (2007) Expression of granulocyte-colony-stimulating factor and its receptor in human Ewing sarcoma cells and patient tumor specimens: potential consequences of granulocyte-colony-stimulating factor administration. Cancer 110(7):1568–1577
Hollmen M, Karaman S, Schwager S, Lisibach A, Christiansen AJ, Maksimow M et al (2016) G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology 5(3):e1115177
Tsuzuki H, Fujieda S, Sunaga H, Noda I, Saito H (1998) Expression of granulocyte colony-stimulating factor receptor correlates with prognosis in oral and mesopharyngeal carcinoma. Cancer Res 58(4):794–800
Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV et al (2014) G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer 110(5):1211–1220
Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE (1999) Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol 155(5):1557–1567
Du H, Zhang H, Zhang Y, Wang Q (2016) Expression of G-CSF and clinical pathological significance in cervical cancer. Zhonghua Yi Xue Za Zhi 96(5):358–360
Tachibana M, Miyakawa A, Tazaki H, Nakamura K, Kubo A, Hata J et al (1995) Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res 55(15):3438–3443
Kasuga I, Ishizuka S, Minemura K, Utsumi K, Serizawa H, Ohyashiki K (2001) Malignant pleural mesothelioma produces functional granulocyte-colony stimulating factor. Chest 119(3):981–983
Hsu DM, Agarwal S, Benham A, Coarfa C, Trahan DN, Chen Z et al (2013) G-CSF receptor positive neuroblastoma subpopulations are enriched in chemotherapy-resistant or relapsed tumors and are highly tumorigenic. Cancer Res 73(13):4134–4146
Agarwal S, Lakoma A, Chen Z, Hicks J, Metelitsa LS, Kim ES et al (2015) G-CSF promotes neuroblastoma tumorigenicity and metastasis via STAT3-dependent cancer stem cell activation. Cancer Res 75(12):2566–2579
Avalos BR, Gasson JC, Hedvat C, Quan SG, Baldwin GC, Weisbart RH et al (1990) Human granulocyte colony-stimulating factor: biologic activities and receptor characterization on hematopoietic cells and small cell lung cancer cell lines. Blood 75(4):851–857
Savarese DM, Valinski H, Quesenberry P, Savarese T (1998) Expression and function of colony-stimulating factors and their receptors in human prostate carcinoma cell lines. Prostate 34(2):80–91
Moon HW, Kim TY, Oh BR, Hwang SM, Kwon J, Ku JL et al (2012) Effects of granulocyte-colony stimulating factor and the expression of its receptor on various malignant cells. Korean J Hematol 47(3):219–224
Kumar J, Fraser FW, Riley C, Ahmed N, McCulloch DR, Ward AC (2014) Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer. Br J Cancer 110(1):133–145
Yang X, Liu F, Xu Z, Chen C, Wu X, Li G et al (2005) Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad Med J 81(955):333–337
Wojtukiewicz MZ, Sierko E, Skalij P, Kaminska M, Zimnoch L, Brekken RA et al (2016) Granulocyte-colony stimulating factor receptor, tissue factor, and VEGF-R bound VEGF in human breast cancer in loco. Adv Clin Exp Med 25(3):505–511
Tachibana M, Miyakawa A, Uchida A, Murai M, Eguchi K, Nakamura K et al (1997) Granulocyte colony-stimulating factor receptor expression on human transitional cell carcinoma of the bladder. Br J Cancer 75(10):1489–1496
Aeed PA, Nakajima M, Welch DR (1988) The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer 42(5):748–759
DuPre SA, Hunter KW (2007) Jr. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol 82(1):12–24
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X et al (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+ Ly6C + granulocytes. Proc Natl Acad Sci USA 107(50):21248–21255
Waight JD, Hu Q, Miller A, Liu S, Abrams SI (2011) Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS ONE 6(11):e27690
Swierczak A, Cook AD, Lenzo JC, Restall CM, Doherty JP, Anderson RL et al (2014) The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunol Res 2(8):765–776
Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K et al (2015) Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA 112(6):E566–E575
Ohmi C, Matsuyama H, Tei Y, Yoshihiro S, Shimabukuro T, Ohmoto Y et al (2003) Granulocyte colony-stimulating factor may promote proliferation of human bladder cancer cells mediated by basic fibroblast growth factor. Scand J Urol Nephrol 37(4):286–291
Chakraborty A, Guha S (2007) Granulocyte colony-stimulating factor/granulocyte colony-stimulating factor receptor biological axis promotes survival and growth of bladder cancer cells. Urology 69(6):1210–1215
Gay AN, Chang S, Rutland L, Yu L, Byeseda S, Naik-Mathuria B et al (2008) Granulocyte colony stimulating factor alters the phenotype of neuroblastoma cells: implications for disease-free survival of high-risk patients. J Pediatr Surg 43(5):837–842
Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M (2002) G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun 297(4):1058–1061
Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M (2006) Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1 + CD11b + cells in cancer animal models. Int Immunol 18(1):1–9
Wang J, Yao L, Zhao S, Zhang X, Yin J, Zhang Y et al (2012) Granulocyte-colony stimulating factor promotes proliferation, migration and invasion in glioma cells. Cancer Biol Ther 13(6):389–400
Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM (2006) Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res 66(16):8026–8036
Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M et al (2009) G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA 106(16):6742–6747
Shaked Y, Tang T, Woloszynek J, Daenen LG, Man S, Xu P et al (2009) Contribution of granulocyte colony-stimulating factor to the acute mobilization of endothelial precursor cells by vascular disrupting agents. Cancer Res 69(19):7524–7528
Voloshin T, Gingis-Velitski S, Bril R, Benayoun L, Munster M, Milsom C et al (2011) G-CSF supplementation with chemotherapy can promote revascularization and subsequent tumor regrowth: prevention by a CXCR4 antagonist. Blood 118(12):3426–3435
Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E et al (2016) Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov 6(6):630–649
Pickup MW, Owens P, Gorska AE, Chytil A, Ye F, Shi C et al (2017) Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte colony stimulating factor secretion in carcinoma cells. Cancer Immunol Res 5(9):718–729
Welte T, Kim IS, Tian L, Gao X, Wang H, Li J et al (2016) Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat Cell Biol 18(6):632–644
Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH et al (2013) Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity 39(3):611–621
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293
Sceneay J, Smyth MJ, Moller A (2013) The pre-metastatic niche: finding common ground. Cancer Metastasis Rev 32(3–4):449–464
Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B (2018) Myeloid-derived suppressor cells: Important contributors to tumor progression and metastasis. J Cell Physiol 233(4):3024–3036
Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM et al (2012) Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res 72(16):3906–3911
Chafe SC, Lou Y, Sceneay J, Vallejo M, Hamilton MJ, McDonald PC et al (2015) Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res 75(6):996–1008
Cao Y, Slaney CY, Bidwell BN, Parker BS, Johnstone CN, Rautela J et al (2014) BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Res 74(18):5091–5102
Morris KT, Castillo EF, Ray AL, Weston LL, Nofchissey RA, Hanson JA et al (2015) Anti-G-CSF treatment induces protective tumor immunity in mouse colon cancer by promoting protective NK cell, macrophage and T cell responses. Oncotarget 6(26):22338–22347
He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y et al (2015) Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res 34:141
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535
Branzk N, Papayannopoulos V (2013) Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol 35(4):513–530
Erpenbeck L, Schon MP (2017) Neutrophil extracellular traps: protagonists of cancer progression? Oncogene 36(18):2483–2490
Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y et al (2016) Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 5(5):e1134073
Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J et al (2016) Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 8(361):361ra138
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K et al (2016) Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 76(6):1367–1380
Yang H, Eaves C, de Lima M, Lee MS, Champlin RE, McMannis JD et al (2006) A novel triple purge strategy for eliminating chronic myelogenous leukemia (CML) cells from autografts. Bone Marrow Transplant 37(6):575–582
Gazitt Y (2001) Recent developments in the regulation of peripheral blood stem cell mobilization and engraftment by cytokines, chemokines, and adhesion molecules. J Hematother Stem Cell Res 10(2):229–236
Togel F, Datta C, Badbaran A, Kroger N, Renges H, Gieseking F et al (2001) Urokinase-like plasminogen activator receptor expression on disseminated breast cancer cells. J Hematother Stem Cell Res 10(1):141–145
Brugger W, Bross KJ, Glatt M, Weber F, Mertelsmann R, Kanz L (1994) Mobilization of tumor cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumors. Blood 83(3):636–640
Pilarski LM, Hipperson G, Seeberger K, Pruski E, Coupland RW, Belch AR (2000) Myeloma progenitors in the blood of patients with aggressive or minimal disease: engraftment and self-renewal of primary human myeloma in the bone marrow of NOD SCID mice. Blood 95(3):1056–1065
Stadtmauer EA, Tsai DE, Sickles CJ, Mick R, Luger SM, Porter DL et al (1999) Stem cell transplantation for metastatic breast cancer: analysis of tumor contamination. Med Oncol 16(4):279–288
Marini O, Costa S, Bevilacqua D, Calzetti F, Tamassia N, Spina C et al (2017) Mature CD10(+) and immature CD10(-) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 129(10):1343–1356
Liu Z, Zhu Y, Wang Y, Fu Q, Fu H, Wang Z et al (2017) Prognostic value of granulocyte colony-stimulating factor in patients with non-metastatic clear cell renal cell carcinoma. Oncotarget 8(41):69961–69971
Wei LX, Chang WL, Guo AT, Tai YH, Sun L, Shi HY (2011) [Expression of granulocyte colony stimulating factor in patients with non-small cell lung cancer and its clinicopathological significance]. Zhonghua Bing Li Xue Za Zhi 40(11):721–725
Nakamura M, Oshika Y, Abe Y, Ozeki Y, Katoh Y, Yamazaki H et al (1997) Gene expression of granulocyte colony stimulating factor (G-CSF) in non-small cell lung cancer. Anticancer Res 17(1B):573–576
Stathopoulos GP, Armakolas A, Tranga T, Marinou H, Stathopoulos J, Chandrinou H (2011) Granulocyte colony-stimulating factor expression as a prognostic biomarker in non-small cell lung cancer. Oncol Rep 25(6):1541–1544
Kawano M, Mabuchi S, Matsumoto Y, Sasano T, Takahashi R, Kuroda H et al (2015) The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci Rep 5:18217
Munstedt K, Hackethal A, Eskef K, Hrgovic I, Franke FE (2010) Prognostic relevance of granulocyte colony-stimulating factor in ovarian carcinomas. Arch Gynecol Obstet 282(3):301–305
Yang XD, Huang P, Wang F, Xu ZK (2014) Expression of granulocyte colony-stimulating factor receptor in rectal cancer. World J Gastroenterol 20(4):1074–1078
Yang Z, Gu JH, Guo CS, Li XH, Yang WC (2017) Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival of epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Oncotarget 8:46414–46424
Ethier JL, Desautels D, Templeton A, Shah PS, Amir E (2017) Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 19(1):2
Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T et al (2016) Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol 23(4):1104–1110
Chen Y, Chen K, Xiao X, Nie Y, Qu S, Gong C et al (2016) Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer 16:320
Bowen RC, Little NAB, Harmer JR, Ma J, Mirabelli LG, Roller KD et al (2017) Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta-analysis. Oncotarget 8(19):32171–32189
Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL et al (2017) Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106:1–7
Marchioni M, Cindolo L, Autorino R, Primiceri G, Arcaniolo D, De Sio M et al (2016) High neutrophil-to-lymphocyte ratio as prognostic factor in patients affected by upper tract urothelial cancer: a systematic review and meta-analysis. Clin Genitourin Cancer 15:343–349
Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ (2017) The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol 115(4):470–479
Ojerholm E, Smith A, Hwang WT, Baumann BC, Tucker KN, Lerner SP et al (2017) Neutrophil-to-lymphocyte ratio as a bladder cancer biomarker: assessing prognostic and predictive value in SWOG 8710. Cancer 123(5):794–801
Meisel A, von Felten S, Vogt DR, Liewen H, de Wit R, de Bono J et al (2016) Severe neutropenia during cabazitaxel treatment is associated with survival benefit in men with metastatic castration-resistant prostate cancer (mCRPC): a post-hoc analysis of the TROPIC phase III trial. Eur J Cancer 56:93–100
Tewari KS, Java JJ, Gatcliffe TA, Bookman MA, Monk BJ (2014) Chemotherapy-induced neutropenia as a biomarker of survival in advanced ovarian carcinoma: an exploratory study of the gynecologic oncology group. Gynecol Oncol 133(3):439–445
Chen Z, Chen W, Wang J, Zhu M, Zhuang Z (2015) Pretreated baseline neutrophil count and chemotherapy-induced neutropenia may be conveniently available as prognostic biomarkers in advanced gastric cancer. Intern Med J 45(8):854–859
Han Y, Yu Z, Wen S, Zhang B, Cao X, Wang X (2012) Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res Treat 131(2):483–490
Kasi PM, Kotani D, Cecchini M, Shitara K, Ohtsu A, Ramanathan RK et al (2016) Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer 16:467
Citron ML (2004) Dose density in adjuvant chemotherapy for breast cancer. Cancer Invest 22(4):555–568
Leonard RC, Mansi JL, Keerie C, Yellowlees A, Crawford S, Benstead K et al (2015) A randomised trial of secondary prophylaxis using granulocyte colony-stimulating factor (‘SPROG’ trial) for maintaining dose intensity of standard adjuvant chemotherapy for breast cancer by the Anglo-Celtic Cooperative Group and NCRN. Ann Oncol 26(12):2437–2441
Renner P, Milazzo S, Liu JP, Zwahlen M, Birkmann J, Horneber M (2012) Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database Syst Rev 10:CD007913
Gray et al (2017) Proceedings of the San Antonio Breast Cancer Symposium, San Antonio
Ye SG, Ding YI, Li L, Yang M, Zhang WJ, Liang AB (2015) Colony-stimulating factors for chemotherapy-related febrile neutropenia are associated with improved prognosis in adult acute lymphoblastic leukemia. Mol Clin Oncol 3(3):730–736
Clamp AR, Ryder WD, Bhattacharya S, Pettengell R, Radford JA (2008) Patterns of mortality after prolonged follow-up of a randomised controlled trial using granulocyte colony-stimulating factor to maintain chemotherapy dose intensity in non-Hodgkin’s lymphoma. Br J Cancer 99(2):253–258
Calip GS, Malmgren JA, Lee WJ, Schwartz SM, Kaplan HG (2015) Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer. Breast Cancer Res Treat 154(1):133–143
Papaldo P, Lopez M, Cortesi E, Cammilluzzi E, Antimi M, Terzoli E et al (2003) Addition of either lonidamine or granulocyte colony-stimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol 21(18):3462–3468
Verma S, Bartlett CH, Schnell P, DeMichele AM, Loi S, Ro J et al (2016) Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist 21(10):1165–1175
Kotto-Kome AC, Fox SE, Lu W, Yang BB, Christensen RD, Calhoun DA (2004) Evidence that the granulocyte colony-stimulating factor (G-CSF) receptor plays a role in the pharmacokinetics of G-CSF and PegG-CSF using a G-CSF-R KO model. Pharmacol Res 50(1):55–58
Watts MJ, Addison I, Long SG, Hartley S, Warrington S, Boyce M et al (1997) Crossover study of the haematological effects and pharmacokinetics of glycosylated and non-glycosylated G-CSF in healthy volunteers. Br J Haematol 98(2):474–479
Acknowledgements
The authors wish to acknowledge grant support from the National Health and Medical Research Council (NHMRC) (APP1080560 – RLA and JAH) and from the National Breast Cancer Foundation (NBCF) of Australia (PS-15-022 – RLA and JAH). Fellowship support from the NBCF (RLA) and scholarship support from Monash University (KAM) are also acknowledged. Olivia Newton-John Cancer Research Institute acknowledges the support of Operational Infrastructure Program of Victorian Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Belinda Yeo and Andrew D. Redfern are joint first authors.
Rights and permissions
About this article
Cite this article
Yeo, B., Redfern, A.D., Mouchemore, K.A. et al. The dark side of granulocyte-colony stimulating factor: a supportive therapy with potential to promote tumour progression. Clin Exp Metastasis 35, 255–267 (2018). https://doi.org/10.1007/s10585-018-9917-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-018-9917-7