Abstract
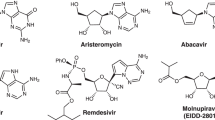
Two strategies for the synthesis of isoxazolidinyl nucleosides as potential antiviral agents are reported: a one-step approach based on 1,3-dipolar cycloaddition of D-lyxosyl nitrone with N,N-dibenzyl-9-vinyl adenine, and a two-step methodology based on the Vorbrüggen nucleosidation of the 5-acetoxyisoxazolidine. The chiral D-lyxosyl nitrone undergoes regioselective 1,3-dipolar cycloadditions with N,N-dibenzyl-9-vinyl adenine and vinyl acetate giving 5-substituted isoxazolidines as a mixture of four diastereoisomers in good yields. The condensation of 5-acetoxyisoxazolidine with silylated uracil, thymine, and N-acetylcytosine proceeded with moderate to good stereoselectivity with the formation of the expected isoxazolidinyl β-and α-nucleosides.
Similar content being viewed by others
References
P. Merino, Curr. Med. Chem. Anti-Infective Agents, 1, 389 (2002).
U. Chiacchio, A. Corsaro, G. Gumina, A. Rescifina, D. Iannazzo, A. Piperno, G. Romeo, and R. Romeo, J. Org. Chem., 64, 9321 (1999).
P. Merino, E. M. Del Alamo, F. Santiago, F. L. Merchan, A. Simon, and T. Tejero, Tetrahedron: Asymmetry, 11, 1543 (2000).
U. Chiacchio, A. Corsaro, D. Iannazzo, A. Pieperno, A. Procopio, A. Rescifina, G. Romeo, R. Romeo, Eur. J. Org. Chem., 1893 (2001).
U. Chiacchio, A. Corsaro, D. Iannazzo, A. Piperno, V. Pistara, A. Procopio, A. Rescifina, G. Romeo, R. Romeo, M. C. R. Siciliano, and E. Valveri, ARKIVOC, xi, 159 (2002).
E. Colacino, A. Converso, A. Ligueri, A. Napoli, C. Siciliano, and G. Sindona, Tetrahedron, 57, 8551 (2001).
U. Chiacchio, A. Corsaro, D. Iannazzo, A. Piperno, V. Pistara, A. Rescifina, R. Romeo, V. Valveri, A. Mastino, and G. Romeo, J. Med. Chem., 46, 3696 (2003).
R. Fischer, A. Drucková, L. Fišera, A. Rybár, C. Hametner, and M. K. Cyrañski, Synlett, 1113 (2002).
R. Fischer, E. Hýrošová, A. Drucková, L. Fišera, C. Hametner, and M. K. Cyrañski, Synlett, 2364 (2003).
J. Kubán, I. Blanáriková, L. Fišera, L. Jarošková, M. Fengler-Veith, V. Jäger, J. Kožíšek, O. Humpa, N. Prónayová, and V. Langer, Tetrahedron, 55, 9501 (1999).
J. Kubán, A. Kolarovič, L. Fišera, V. Jäger, O. Humpa, and N. Prónayová, P. Ertl, Synlett, 1862 (2001).
J. Kubán, A. Kolarovič, L. Fišera, V. Jäger, O. Humpa, and N. Prónayová, Synlett, 1862 (2001).
I. Blanáriková-Hlobilová, Z. Kopaničáková, L. Fišera, M. K. Cyrañski, P. Salanski, J. Jurczak, and N. Prónayová, Tetrahedron, 59, 3333 (2003).
B. Dugovič, T. Wiesenganger, L. Fišera, C. Hametner, and N. Prónayová, Heterocycles, 65, 591 (2005).
B. Dugovič, L. Fišera, M. K. Cyranski, C. Hametner, N. Prónayová, and M. Obranec, Helvetica Chim. Acta, 88, 1432 (2005).
A. Dondoni, S. Franco, F. Junquera, F. L. Merchan, P. Merino, and T. Tejero, Synth. Commun., 23, 2537 (1994).
P. Merino, S. Franco, F. L. Merchan, P. Romero, T. Tejero, and S. Uriel, Tetrahedron: Asymmetry, 14, 3731 (2003).
R. Fischer, A. Drucková, L. Fišera, and C. Hametner, ARKIVOC, viii, 80 (2002).
H. Vorbrüggen, K. Krolikiewicz, and B. Bennua, Chem. Ber., 114, 1234 (1981).
P. Merino, S. Franco, F. L. Merchan, and T. Tejero, J. Org. Chem., 65, 5575 (2000).
U. Chiacchio, A. Rescifina, A. Corsaro, V. Pistara, G. Romeo, and R. Romeo, Tetrahedron: Asymmetry, 11, 2045 (2000).
U. Chiacchio, A. Corsaro, D. Iannazzo, A. Piperno, V. Pistara, A. Rescifina, R. Romeo, G. Sindona, and G. Romeo, Tetrahedron: Asymmetry, 14, 2717 (2003).
U. Chiacchio, D. Iannazzo, A. Piperno, R. Romeo, G. Romeo, A. Rescifina, and M. Saglimbeni, Bioorg. Med. Chem., 14, 955 (2006).
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. E. Lukevics on the occasion of his 70th birthday
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 01, pp. 14–21, Januvary, 2007.
Rights and permissions
About this article
Cite this article
Hýrošová, E., Fišera, L., Jame, R.MA. et al. Diastereoselective synthesis of some carbocyclic 2′-oxa-3′-aza-nucleosides. Chem Heterocycl Compd 43, 10–17 (2007). https://doi.org/10.1007/s10593-007-0002-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10593-007-0002-4