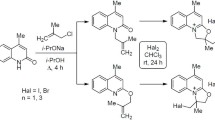
Independently of the reaction conditions N-allylisatin forms only the 2,3-dibromo derivative upon treatment with molecular bromine in contrast to N-allyl-substituted quinolin- or pyrid-2-ones which readily undergo halocyclization to the corresponding 2-bromomethyl oxazoles.
Similar content being viewed by others
References
I. V. Ukrainets, Liu Yangyang, N. L. Bereznyakova, and A. V. Turov, Khim. Geterotsikl. Soedin., 1539 (2009). [Chem. Heterocycl. Comp., 45, 1236 (2009)].
F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry. Pt. B: Reactions and Synthesis, 5th ed., Springer USA (2007), p. 310. Online version available.
J. W. Chern, C. Y. Shiau, and G. Y. Lu, Bioorg. Med. Chem. Lett., 1, 571 (1991).
M. D'hooghe, J. Baele, J. Contreras, M. Boelens, and N. De Kimpe, Tetrahedron Lett., 49, 6039 (2008).
T. Hu, M. Shen, Q. Chen, and C. Li, Org. Lett., 8, 2647 (2006).
T. M. Ugurchieva, A. V. Lozanova, M. V. Zlokazov, and V. V. Veselovsky, Russian Chem. Bull., Int. Ed., 56, 1544 (2007).
V. N. Britsun, A. N. Esipenko, V. I. Staninets, and M. O. Lozinskii, Khim. Geterotsikl. Soedin., 1114 (2005). [Chem. Heterocycl. Comp., 41, 948 (2005)].
I. V. Ukrainets, L. V. Sidorenko, O. V. Gorokhova, S. V. Shishkina, and A. V. Turov, Khim. Geterotsikl. Soedin., 736 (2007). [Chem. Heterocycl. Comp., 43, 617 (2007)].
I. V. Ukrainets, N. L. Bereznyakova, O. V. Gorokhova, A. V. Turov, ad S. V. Shishkin, Khim. Geterotsikl. Soedin., 1180 (2007). [Chem. Heterocycl. Comp., 43, 1001 (2007)].
I. V. Ukrainets, N. L. Bereznyakova, V. A. Parshikov, and A. V. Turov, Khim. Geterotsikl. Soedin., 1496 (2007). [Chem. Heterocycl. Comp., 43, 1296 (2007)].
G. I. Zhungietu and M. A. Rekhter, Isatin and its Derivatives [in Russian], Shtiintsa, Kishinev (1977), p. 33.
H.-B. Burgi and J. D. Dunitz, Structure Сorrelation, Vol. 2, VCH, Weinheim (1994), p. 741.
M. H. Palmer, A. J. Blake, and R. O. Gould, Chem. Phys., 115, 219 (1987).
J. Zukerman-Schpector, E. E. Castellano, A. D. C. Pinto, J. F. M. da Silva, and M. T. F. C. Barcellos, Acta Crystallogr., C48, 760 (1992).
J. Zukerman-Schpector, A. D. C. Pinto, J. F. M. da Silva, and M. T. F. C. Barcellos, Acta Crystallogr., C51, 675 (1995).
G. Miehe, P. Susse, V. Kupcik, E. Egert, M. Nieger, G. Kunz, R. Gerke, B. Knieriem, M. Niemeyer, and W. Luttke, Angew. Chem., Int. Ed., 30, 964 (1991).
Yu. V. Zefirov, Kristallografiya, 42, 936 (1997).
S. M. Khripak, M. V. Slivka, R. V. Vilkov, R. N. Usenko, and V. G. Lendel, Khim. Geterotsikl. Soedin., 922 (2007). [Chem. Heterocycl. Comp., 43, 781 (2007)].
M. V. Slivka, Dis. Cand. Chem. Sci. [in Russian], Kiev (2001).
M. V. Slivka, S. M. Khripak, V. N. Britsun, and V. I. Staninets, Zh. Org. Khim., 36, 1064 (2000).
D. G. Kim, V. V. Avdin, and L. V. Garrilova, Khim. Geterotsikl. Soedin., 1130 (1997). [Chem. Heterocycl. Comp., 33, 986 (1997)].
D. G. Kim, Khim. Geterotsikl. Soedin., 566 (1998). [Chem. Heterocycl. Comp., 34, 505 (1998).
Y. G. Kim and J. K. Cha, Tetrahedron Lett., 30, 5721 (1989).
L. A. Paquette, G. D. Crouse, and A. K. Sharma, J. Am. Chem. Soc., 102, 3972 (1980).
I. V. Ukrainets, N. L. Bereznyakova, V. A. Parshikov, and A. V. Turov, Khim. Geterotsikl. Soedin., 1496 (2007). [Chem. Heterocycl. Comp., 43, 1269 (2007)].
A. F. Pozharskii, Theoretical Basis for the Chemistry of Heterocycles [in Russian], Khimiya, Moscow (1985), p. 158.
I. V. Ukrainets, L. V. Sidorenko, O. V. Gorokhova, N. L. Bereznyakova, and S. V. Shishkina, Khim. Geterotsikl. Soedin., 1502 (2006). [Chem. Heterocycl. Comp., 42, 1296 (2006)].
G. M. Sheldrick, SHELXTL PLUS. PC Version. A System of Computer Programs for the Determination of Crystal Structure from X-ray Diffraction Data. Rev. 5.1 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
For Communication 169 see [1].
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 10, pp. 1546-1553, October, 2009.
Rights and permissions
About this article
Cite this article
Ukrainets, I.V., Bereznyakova, N.L., Gorokhova, O.V. et al. 4-hydroxy-2-quinolones 170*. synthesis and bromination of N-allylisatin. Chem Heterocycl Comp 45, 1241–1247 (2009). https://doi.org/10.1007/s10593-010-0413-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-010-0413-5