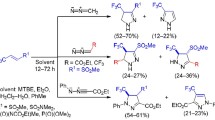
Cycloaddition reactions of 3,3,3-trifluoropropene derivatives containing an alkoxycarbonyl, sulfonyl, sulfoximine, or sulfamide substituent in position 1 with ethyl isocyanoacetate proceed with the formation of 3-(trifluoromethyl)-2,3-dihydro-1H-pyrroles, whereas their reactions with tosylmethyl isocyanide lead to the formation of 4-(trifluoromethyl)-1H-pyrroles.

Similar content being viewed by others

Notes
Hereinafter in the Experimental, the signals of the major isomer are indicated with an asterisk (*), signals of both isomers are indicated with two asterisks (**).
References
(a) Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. RSC Adv. 2015, 5, 15233. (b) Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. Eur. J. Med. Chem. 2000, 35, 499.
(а) Bellina, F.; Rossi, R. Tetrahedron 2006, 62, 7213. b Zhang, Y.; Ran, C.; Zhou, G.; Sayre, L. M. Bioorg. Med. Chem. 2007, 15, 1868. c Kawai, H.; Yuan, Z.; Kitayama, T.; Tokunaga, E.; Shibata, N. Angew. Chem., Int. Ed. 2013, 52, 5575. d Medran, N. S.; La-Venia, A.; Testero, S. A. RSC Adv. 2019, 9, 6804. e Meninno, S.; Capobianco, A.; Peluso, A.; Lattanzi, A. Green Chem. 2015, 17, 2137.
(a) Elbein, A. D. Annu. Rev. Biochem. 1987, 56, 497. (b) Winchester, B.; Fleet, G. W. Glycobiology 1992, 2, 199. (c) Hensler, M. E.; Bernstein, G.; Nizet, V.; Nefzi, A. Bioorg. Med. Chem. Lett. 2006, 16, 5073. (d) Li, X.; Li, Y.; Xu, W. Bioorg. Med. Chem. 2006, 14, 1287. (e) Malawska, B. Curr. Top. Med. Chem. 2005, 5, 69. (f) Colandrea, V. J.; Legiec, I. E.; Huo, P.; Yan, L.; Hale, J. J.; Mills, S. G.; Bergstrom, J.; Card, D.; Chebret, G.; Hajdu, R.; Keohane, C. A.; Milligan, J. A.; Rosenbach, M. J.; Shei, G.-J.; Mandala, S. M. Bioorg. Med. Chem. Lett. 2006, 16, 2905. (g) Yan, L.; Budhu, R.; Huo, P.; Lynch, C. L.; Hale, J. J.; Mills, S. G.; Hajdu, R.; Keohane, C. A.; Rosenbach, M. J.; Milligan, J. A.; Shei, G.-J.; Chrebet, G.; Bergstrom, J.; Card, D.; Mandala, S. M. Bioorg. Med. Chem. Lett. 2006, 16, 3564. (h) Barrett, D. G.; Catalano, J. G.; Deaton, D. N.; Hassell, A. M.; Long, S. T.; Miller, A. B.; Miller, L. R.; Ray, J. A.; Samano, V.; Shewchuk, L. M.; Wells-Knecht, K. J.; Willard, D. H., Jr.; Wright, L. L. Bioorg. Med. Chem. Lett. 2006, 16, 1735. (i) Tran, J. A.; Chen, C. W.; Jiang, W.; Tucci, F. C.; Fleck, B. A.; Marinkovic, D.; Arellano, M.; Chen, C. Bioorg. Med. Chem. Lett. 2007, 17, 5165.
(a) Gakh, A. A.; Shermolovich, Yu. G. Curr. Top. Med. Chem. 2014, 14, 952. (b) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359. (c) Prakash, G. S.; Chacko, S. Curr. Opin. Drug Discovery Dev. 2008, 11, 793. (d) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(a) Kawai, H.; Yuan, Z.; Kitayama, T.; Tokunaga, E.; Shibata, N. Angew. Chem., Int. Ed. 2013, 52, 5575. (b) Marrec, O.; Christophe, C.; Billard, T.; Langlois, B.; Vors, J.-P.; Pazenoc, S. Adv. Synth. Catal. 2010, 352, 2825. (с) Kawai, H.; Kitayama, T.; Tokunaga, E.; Takashi, M.; Sato, S.; Shiro, M.; Shibata. N. Chem. Commun. 2012, 48, 4067.
(a) Zhu, Z.; Guo, Y.; Wang, X.; Wu, F.; Wu, Y. J. Fluorine Chem. 2017, 195, 102. (b) Tang, D.-D.; Wang, Y.; Wang, J.-R.; Xu, P.-F. Tetrahedron Lett. 2014, 55, 4133. (c) Muzalevksiy, V. M.; Shastin, A. V.; Balenkova, E. S.; Haufe, G.; Nenajdenko, V. G. Synthesis 2009, 3905.
Kirk, K. L. In Fluorinated Heterocyclic Compounds: Synthesis, Chemistry and Applications; Petrov, C. A., Ed.; John Wiley & Sons: Hoboken, 2009, p. 91.
(a) Larionov, O. V.; de Meijere, A. Angew. Chem., Int. Ed. 2005, 44, 5664. (b) Lygin, A. V.; Larionov, O. V.; Korotkov, V. S.; de Meijere, A. Chem.–Eur. J. 2009, 15, 227. (c) Yang, X.-G.; John, R.; Seitz, G. Arch. Pharm. 1991, 324, 923. (d) Chen, C.-Y.; Bocian, D. F.; Lindsey, J. S. J. Org. Chem. 2014, 79, 1001. (e) Taguchi, T.; Tomizawa, G.; Kawara, A.; Nakajima, M.; Kobayashi, Y. J. Fluorine Chem. 1988, 40, 171. (f) Ponce, A.; Alonso, I.; Adrio, J.; Carretero, J. C. Chem.–Eur. J. 2016, 22, 4952. (g) Feng, B.; Lu, L.-Q. Chen, J.-R.; Feng, G.; He, B.-Q.; Lu, B.; Xiao, W.-J. Angew. Chem., Int. Ed. 2018, 57, 5888. (h) Cheng, F.; Kalita, S. J.; Zhao, Z.-N.; Yang, X.; Zhao, Y.; Schneider, U.; Shibata, N.; Huang, Y. Y. Angew. Chem., Int. Ed. 2019, 58, 16637. (i) Llamas, T.; Arrayás, R. G.; Carretero, J. C. Synthesis 2007, 950.
(a) Markitanov, Yu. N.; Timoshenko, V. M.; Shermolovich, Yu. G.; Mykhalchuk, V. L.; Grafova, I. A.; Grafov, A. V. Chem. Heterocycl. Compd. 2016, 52, 503. [Khim. Geterotsikl. Soedin. 2016, 52, 503.] (b) Markitanov, Yu. M.; Timoshenko, V. M.; Shermolovich, Yu. G. Chem. Heterocycl. Compd. 2018, 54, 89. [Khim. Geterotsikl. Soedin, 2018, 54, 89.] (c) Markitanov, Yu. M.; Timoshenko, V. M.; Rudenko, T. V.; Rusanov, E. B.; Shermolovich, Yu. G. J. Sulfur Chem. 2019, 40, 629.
(a) Grigg, R.; Lansdell, M. I.; Thornton-Pett, M. Tetrahedron 1999, 55, 2025. (b) Saegusa, T.; Ito, Y.; Kinoshita, H.; Tomita, S. J. Org. Chem. 1971, 36, 3316.
(a) Van Leusen, A. M.; Siderius, H.; Hoogenboom, B. E.; Van Leusen, D. Tetrahedron Lett. 1972, 52, 5337. (b) Tandon, V. K.: Rai, S. Sulfur Rep. 2003, 24, 307. (c) Ma, Z.; Ma, Z.; Zhang, D. Molecules 2018, 23, 2666. (d) Aoyagi, K.; Haga, T.; Toi, H.; Aoyama, Y.; Mizutani, T.; Ogoshi, H. Bull. Chem. Soc. Jpn. 1997, 70, 937. (e) Leroy, J. J. Fluorine Chem. 1991, 53, 61. (f) Aoyagi, K.; Haga, T.; Toi, H.; Aoyama, Y.; Ogoshi, H. Chem. Lett. 1988, 17, 1891.
(a) Winters, M. P.; Sui, Z.; Wall, M.; Wang, Y.; Gunnet, J.; Leonard J.; Hua, H.; Yan, W.; Suckow, A.; Bell, A.; Clapper, W.; Jenkinson, C.; Haug, P.; Koudriakova, T.; Huebert, N.; Murray, W. V. Bioorg. Med. Chem. Lett. 2018, 28, 841. (b) Monteiro, J. L.; Carneiro, P. F.; Elsner, P.; Roberge, D. M.; Wuts, P. G. M.; Kurjan, K. C.; Gutmann, B.; Kappe, C. O. Chem.–Eur. J. 2017, 23, 176. (c) Baar, M.; Blechert, S. Chem.–Eur. J. 2015, 21, 526.
(a) Walter, H. Z. Naturforsch., B: J. Chem. Sci. 2008, 63, 351. (b) Eberle, М.; Val'ter, Kh. RF Patent № 2264388C2, 1999. b Brown, D. G.; Diehl, R. E.; Lowen, G. T.; Wright, D. P., Jr.; Kukel, C. F.; Herman, R. A.; Addor, R. W. US Patent 5162308A, 1992.
(a) Black, D. St. C.; Bowyer, M. C.; Kumar, N. Tetrahedron 1997, 53, 8573. (b) Gribble, G. W.; Hoffman, J. H. Synthesis 1977, 859.
Zhang, H.-H.; Shen, W.; Lu, L. Tetrahedron Lett. 2018, 59, 1042.
Uno, H.; Tanaka, M.; Inoue, T.; Ono, N. Synthesis 1999, 471.
(a) Hodges, J. A.; Raines, R. T. J. Am. Chem. Soc. 2005, 127, 15923. (b) Verhoork, S. J. M.; Killoran, P. M.; Coxon, C. R. Biochemistry 2018, 57, 6132. (c) Chaume, G.; Van Severen, M.-C.; Marinkovic, S.; Brigaud, T. Org. Lett. 2006, 8, 6123. (d) Del Valle, J. R.; Goodman, M. Angew. Chem., Int. Ed. 2002, 41, 1600. (e) Kondratov, I. S.; Dolovanyuk, V. G.; Tolmachova, N. A.; Gerus, I. I.; Bergander, K.; Fröhlich, R.; Haufe, G. Org. Biomol. Chem. 2012, 10, 8778.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(3), 253–260
Rights and permissions
About this article
Cite this article
Markitanov, Y.N., Timoshenko, V.M., Rusanov, E.B. et al. [3+2] Cycloaddition reactions of 1-substituted 3,3,3-trifluoropropenes with isonitriles – synthesis of pyrroles and pyrrolines. Chem Heterocycl Comp 57, 253–260 (2021). https://doi.org/10.1007/s10593-021-02901-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02901-x