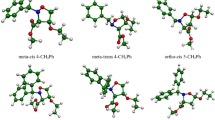
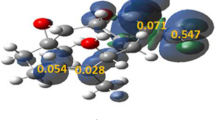
The chemo-, regio-, and stereoselectivity of the hetero-Diels–Alder reaction between 3-benzoylpyrrolo[1,2-c][1,4]benzoxazine-1,2,4-trione and vinyl acetate, has been investigated within molecular electron density theory at the B3LYP/6-311(d,p) level of theory. The conceptual density functional theory reactivity indices, as well as the activation and reaction energies have been analyzed, and five possible reaction paths for this cycloaddition reaction, resulting from the presence of three heterodiene frameworks in the molecule of the diene reactant have been studied. The conceptual density functional theory analysis indicates that vinyl acetate acts as a nucleophile and 3-benzoylpyrrolo[1,2-c][1,4]benzoxazine-1,2,4-trione acts as a strong electrophile. The computed activation and reaction energies reveal that that this reaction is chemo-, stereo-, and regioselective, which is consistent with the experimental findings. Topological electron localization function analysis was used to look into how the electron densities were reorganized along the preferred reaction path. According to this analysis, the reaction takes place through a two-stage one-step mechanism.







Similar content being viewed by others
References
Kasatkina, S. O.; Titov, M. S.; Stepanova, E. E.; Dmitriev, M. V.; Maslivets, A. N. Russ. J. Org. Chem. 2018, 54, 626.
Moroz, A. A.; Antonov, D. I.; Dmitriev, M. V.; Maslivets, A. N. Russ. J. Org. Chem. 2022, 58, 282.
Stepanova, E. E.; Dmitriev, M. V.; Maslivets, A. N. Tetrahedron Lett. 2020, 61, 151595.
Vinogradov, M. G.; Turova, O. V.; Zlotin, S. G. Adv. Synth. Catal. 2021, 363, 1466.
Kącka-Zych, A.; Jasinski, R. J. Mol. Graphics Modell. 2020, 101, 107714.
Ciulla, M. G.; Zimmermann, S.; Kumar, K. Org. Biomol. Chem. 2019, 17, 413.
Khorief Nacereddine, A.; Merzoud, L.; Morell, C.; Chermette, H. J. Comput. Chem. 2021, 42, 1296.
Ouahdi, Z.; Ourhriss, N.; Aitouna, A. O.; Barhoumi, A.; Belghiti, M. E.; Moubarik, A.; El Alaoui El Abdallaoui, H.; El Idrissi, M.; Zeroual, A. Theor. Chem. Acc. 2022, 141, 50.
Zeroual, A.; Ríos-Gutiérrez, M.; El Idrissi, M.; El Alaoui El Abdallaoui, H.; Domingo, L. R. Int. J. Quantum Chem. 2019, 119, e25980.
Siadati, S. A.; Rezazadeh, S. Sci. Radices 2022, 1, 46.
Żmigrodzka, M.; Sadowski, M.; Kras, J.; Dresler, E.; Demchuk, O. M.; Kula, K. Sci. Radices 2022, 1, 26.
El Idrissi, M.; El Ghozlani, M.; Eşme, A.; Ríos-Gutiérrez, M.; Ouled Aitouna, A.; Salah, M.; El Abdallaoui, H. E. A.; Zeroual, A.; Mazoir, N.; Domingo, L. R. Organics 2021, 2, 1.
Zeroual, A.; Ríos-Gutiérrez, M.; El Ghozlani, M.; El Idrissi, M.; Ouled Aitouna, A.; Salah, M.; El Alaoui El Abdallaoui, H.; Domingo, L. R. Theor. Chem. Acc. 2020, 139, 37.
Zeroual, A.; Ríos-Gutiérrez, M.; Salah, M.; El Alaoui El Abdallaoui, H.; Domingo, L. R. J. Chem. Sci. 2019, 131, 75.
Zahnoune, R.; Asserne, F.; Ourhriss, N.; Ouald Aytouna, A.; Barhoumi, A.; Hakmaoui Y.; Belghiti, M. E.; Abouricha, S.; El ajlaoui, R.; Zeroual, A. J. Mol. Struct. 2022, 1269, 133630.
El Ghozlani, M.; Barhoumi, A.; Elkacmi, R.; Ouled Aitouna, A.; Zeroual, A.; El Idrissi, M. Chem. Africa 2020, 3, 901.
Ma, W.-Y.; Montinho-Inacio, E.; Iorga, B. I.; Retailleau, P.; Moreau, X.; Neuville, L.; Masson, G. Adv. Synth. Catal. 2022, 364, 1708.
Zheng, Y.; Qian, S.; Xu, P.; Ma, T.; Huang, S. Adv. Synth. Catal. 2022, 364, 3800.
Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J. Med. Chem. 2014, 57, 5845.
Stepanova, E. E.; Maslivets, A. N. Russ. J. Org. Chem. 2016, 52, 879.
Domingo, L. R. Molecules 2016, 21, 1319.
Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049.
Mohammad-Salim, H. A.; Basheer, H. A.; Abdallah, H. H.; Zeroual, A.; Abdi Jamila, L. New J. Chem. 2021, 45, 262.
Salah, M.; Belghiti, M. E.; Aitouna, A. O.; Zeroual, A.; Jorio, S.; El Alaoui Abdellaoui, H.; El Hadki, H.; Marakchi, K.; Komiha, N. J. Mol. Graphics Modell. 2021, 102, 107763.
Abbiche, K.; Mohammad-Salim, H.; Salah, M.; Mazoir, N.; Zeroual, A.; El Alaoui El Abdallaoui, H.; El Hammadi, A.; Hilali, M.; Abdallah, H. H.; Hochlaf, M. Theor. Chem. Acc. 2020, 139, 148.
Salah, M.; Zeroual, A.; Jorio, S.; El Hadki, H.; Kabbaj, O.; Marakchi, K.; Komiha, N. J. Mol. Graphics Modell. 2020, 94, 107458.
Zoubir M.; Elalaoui Belghiti, M.; El idrissi, M.; Zeroual, A. Theor. Chem. Acc. 2022, 141, 8.
Id El Mouden, O.; Belghiti, ME.; Mizeb, K.; El Ghozlani, M.; Matine, A.; Batah, A.; Bammou, L.; Rakib, E. M.; Zeroual, A.; Belkhaouda, M.; Belaaouad, S.; Elalaoui-Elabdallaoui, H. Results Chem. 2023, 5, 100641.
Becke, A. D. J. Chem. Phys. 1992, 96, 2155.
McLean A. D.; Chandler, G. S. J. Chem. Phys. 1980, 72, 5639.
Domingo, L. R; Pérez, P.; Sáez, J. A. RSC Adv. 2013, 3, 1486.
Hratchian, H. P.; Schlegel, H. B. J. Chem. Theory Comput. 2005, 1, 61.
Domingo, L. R.; Chamorro, E.; Pérez, P. J. Org. Chem. 2008, 73, 4615.
Parr, R. G.; Pearson, R. G. J. Am. Chem. Soc. 1983, 105, 7512.
Gupta, V. P. Principles and Applications of Quantum Chemistry; Elsevier: Amsterdam, 2016, p. 359.
Domingo L. R.; Ríos-Gutiérrez M.; Pérez P. Molecules 2016, 21, 748.
Jaramillo, P.; Domingo, L. R.; Chamorro, E.; Pérez, P. J. Mol. Struct.: THEOCHEM 2008, 865, 68.
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R. Tetrahedron 2002, 58, 4417.
Silvi, B. J. Mol. Struct. 2002, 614, 3.
Fuster, F.; Sevin, A.; Silvi, B. J. Phys. Chem. A 2000, 104, 852.
Silvi, B.; Savin, A. Nature 1994, 371, 683.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(3), 165–170
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ameur, S., Barhoumi, A., Ríos-Gutiérrez, M. et al. A MEDT study of the mechanism and selectivity of the hetero-Diels–Alder reaction between 3-benzoylpyrrolo[1,2-c][1,4]-benzoxazine-1,2,4-trione and vinyl acetate. Chem Heterocycl Comp 59, 165–170 (2023). https://doi.org/10.1007/s10593-023-03178-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03178-y