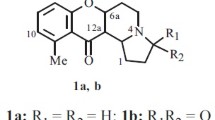
An oxazine ring was annelated to benzopyran-4-one and benzopyran-2-one cores by reacting 7-hydroxyisoflavones and 7-hydroxycoumarins with lupinylamine and formalin. The new derivatives 9,10-dihydro4H,8 H-chromeno[8,7-e][1,3]oxazin-4-one and 9,10-dihydro-2 H,8 H-chromeno[8,7-e][1,3]oxazin-2-one containing a lupinine moiety in the 9-position were prepared.
Similar content being viewed by others
References
A. A. Semenov, Outline of the Chemistry of Natural Compounds [in Russian], Novosibirsk, 2000.
A. S. Sadykov, Kh. A. Aslanov, and Yu. K. Kushmuradov, Alkaloids of the Quinolizidine Class. Chemistry, Stereochemistry, and Biogenesis [in Russian], Moscow, 1975.
R. T. Tlegenov, Kh. Kh. Khaitbaev, Z. Tilyabaev, D. N. Dalimov, A. A. Abduvakhabov, and K. U. Uteniyazov, Khim. Prir. Soedin., 64 (1991).
A. A. Abduvakhabov, R. T. Tlegenov, D. N. Dalimov, Kh. Kh. Khaitbaev, Z. Tilyabaev, F. G. Kamaev, and K. U. Uteniyazov, Uzb. Khim. Zh., 43 (1991).
M. I. Kabachnik, A. S. Sadykov, N. N. Godovikov, Kh. A. Aslanov, A. A. Abduvakhabov, and K. Toremuratov, Izv. Akad. Nauk SSSR, Ser. Khim., 952 (1972).
A. A. Abduvakhabov, R. T. Tlegenov, Kh. Kh. Khaitbaev, G. I. Vaizburg, D. N. Dalimov, and K. U. Uteniyazov, Khim. Prir. Soedin., 75 (1990).
F. Z. Galin, V. G. Kartsev, O. B. Flekhter, G. V. Giniyatullina, and G. A. Tolstikov, Khim. Prir. Soedin., 467 (2004).
M. Casagrande, N. Basilico, S. Parapini, S. Romeo, D. Taramelli, and A. Sparatore, Bioorg. Med. Chem., 16, 6813 (2008).
E. A. Dikusar, N. G. Kozlov, R. T. Tlegenov, and V. I. Potkin, Khim. Rastit. Syr′ya, 111 (2008).
R. T. Tlegenov, Khim. Rastit. Syr′ya, 69 (2007).
A. A. Abduvakhabov, Kh. A. Aslanov, N. N. Godovikov, M. I. Kabachnik, A. S. Sadykov, andV. U. Rakhmagulina, Izv. Akad. Nauk SSSR, Ser. Khim., 946 (1972).
I. V. Nagorichna, M. M. Garazd, Ya. L. Garazd, and V. P. Khilya, Khim. Prir. Soedin., 14 (2007).
S. P. Bondarenko, M. S. Frasinyuk, and V. P. Khilya, Khim. Geterotsikl. Soedin. 672 (2010).
S. P. Bondarenko, M. S. Frasinyuk, and V. P. Khilya, Khim. Prir. Soedin., 418 (2009).
A. Pelter and S. Foot, Synthesis, 5, 326 (1976).
A. Levai, W. Adam, R. T. Fell, R. Gessner, T. Patonay, A. Simon, and G. Toth, Tetrahedron, 54, 13105 (1998).
A. Levai, T. Patonai, A. Szekely, and W. Adam, J. Heterocycl. Chem., 37, 1065 (2000).
D. K. Bharadwaj, R. Murari, T. R. Seshadri, and R. Singh, Phytochemistry, 15, No. 3, 352 (1976).
J. Walker, J. Am. Chem. Soc., 80, 645 (1958).
N. R. Krishnaswamy, T. R. Seshadri, and B. R. Sharma, Indian J. Chem., 4, 120 (1966).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2012, pp. 212–214.
Rights and permissions
About this article
Cite this article
Bondarenko, S.P., Frasinyuk, M.S., Galaev, A.I. et al. New flavonoid-containing derivatives of lupinine. Chem Nat Compd 48, 234–237 (2012). https://doi.org/10.1007/s10600-012-0212-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-012-0212-6