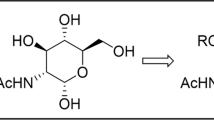
N-Acetylmuramyl-L-alanyl-D-isoglutamine α-phenylglycoside was synthesized from α-phenyl-D-glucosaminide peracetate, which was obtained by fusing β-D-glucosamine pentaacetate with phenol and zinc chloride followed by deacetylation. α-Phenyl-N-acetylglucosaminide was converted in two steps into 4,6-O-isopropylidene-N-acetyl-D-muramic acid α-phenylglycoside, which was condensed with L-Ala-D-iGln benzyl ether using the activated ester method. Sequential removal of glycopeptide protecting groups via acid hydrolysis and catalytic hydrogenolysis gave the target product.
Similar content being viewed by others
References
C. Ogawa, Y.-J. Liu, and K. S. Kobayashi, Curr. Bioact. Compd., 7, 180 (2011).
T. M. Andronova and B. V. Pinegin, Venerologiya, 11 (2006).
A. E. Zemlyakov, V. N. Tsikalova, V. V. Tsikalov, V. Ya. Chirva, E. L. Mulik, and O. V. Kalyuzhin, Bioorg. Khim., 32, 424 (2006).
A. E. Zemlyakov, V. V. Tsikalov, O. V. Kalyuzhin, V. O. Kur′yanov, and V. Ya. Chirva, Bioorg. Khim., 29, 316 (2003).
P. Lefrancier, M. Derrien, I. Lederman, F. Nief, J. Choay, and E. Lederer, Int. J. Pept. Protein Res., 11, 289 (1978).
A. E. Zemlyakov, V. V. Tsikalov, V. O. Kur′yanov, V. Ya. Chirva, and N. V. Bovin, Bioorg. Khim., 27, 439 (2001).
A. E. Zemlyakov, V. N. Tsikalova, V. V. Tsikalov, and V. Ya. Chirva, Zh. Org. Farm. Khim., 2 (3), 17 (2004).
A. E. Zemlyakov, V. N. Tsikalova, V. V. Tsikalov, V. Ya. Chirva, E. L. Mulik, and O. V. Kalyuzhin, Bioorg. Khim., 31, 637 (2005).
O. V. Kalyuzhin, A. E. Zemlyakov, E. V. Kalyuzhina, M. V. Shkalev, and M. V. Nelyubov, Byull. Eksp. Biol. Med., 134, 186 (2002).
W. Bernard, J. Org. Chem., 31, 2505 (1966).
G. Wei, X. Lv, and Y. Du, Carbohydr. Res., 343, 3096 (2008).
K. Yamamoto, M. Fujinaga, and Y. Matsushima, Bull. Chem. Soc. Jpn., 36, 1275 (1963).
P. Lefrancier and E. Lederer, Prog. Chem. Org. Nat. Prod., 40, 1 (1981).
A. S. Shashkov, A. Yu. Evstigneev, and V. A. Derevitskaya, Bioorg. Khim., 4, 1495 (1978).
A. E. Zemlyakov, V. O. Kur′yanov, V. Ya. Chirva, and T. M. Andronova, Bioorg. Khim., 13, 1575 (1987).
S. S. Pertel, E. S. Kakayan, S. A. Seryi, and A. W. McDonagh, Carbohydrate Chemistry: Proven Synthetic Methods, Vol. 4, CRC Press, Boca Raton, 2017, p. 125.
Acknowledgment
The work was performed using equipment (Thermo Scientific MS/MS TSQ Quantum Access MAX) obtained through the Development Program of V. I. Vernadsky Crimean Federal University for 2015-2024.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2020, pp. 173–176.
Rights and permissions
About this article
Cite this article
Zemlyakov, A.E., Tsikalova, V.N. & Tsikalov, V.V. Synthesis of N-Acetylmuramyl-L-Alanyld- Isoglutamine α-Phenylglycoside. Chem Nat Compd 56, 193–196 (2020). https://doi.org/10.1007/s10600-020-02986-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-02986-4