Abstract
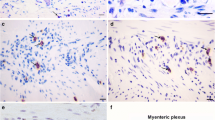
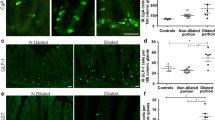
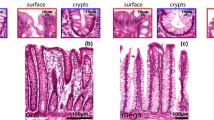
Neuronal destruction has been considered the hallmark of pathogenic mechanisms in chagasic megacolon. Characterization of neuropeptides in the enteric nervous system from chagasic patients with megacolon could elucidate some aspects of the development of this syndrome. In the present work we demonstrate the changes in expression of neuropeptides and neurochemical markers present in neuronal plexuses from the colons of chagasic patients with megacolon. Sections of frozen tissue samples were immunohistochemically labeled for anticalretinin, cChaT, substance P, VIP, NOS, and NPY. Immunoreactivity was observed using a confocal microscope. Our results demonstrate that in chagasic patients with megacolon, inhibitory motor neurons (VIP and NOS immunoreactive) are preferentially destroyed by Trypanosoma cruzi and/or the inflammatory process. These results suggest a selective destruction of enteric neurons in the colon of chagasic patients with megacolon, pointing to an important discovery in the mechanism of pathogenesis of Chagas’ disease.

Similar content being viewed by others
References
Nowicki MJ, Chinchilla C, Corado L, Matsuoka L, Selby R, Steurer F, Mone T, Mendez R, Aswad S (2006) Prevalence of antibodies to trypanosoma cruzi among solid organ donors in southern California: a population at risk. Transplantation 81:477–479
Chapadeiro E (1999) Clinical evolution and morbi-mortality in chagas disease. Mem Inst Oswaldo Cruz 94 (Suppl 1):309–310
Prata A (2001) Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 1:92–100
Koberle F (1968) Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv Parasitol 6:63–116
Adad SJ, Cancado CG, Etchebehere RM, Teixeira VP, Gomes UA, Chapadeiro E, Lopes ER (2001) Neuron count reevaluation in the myenteric plexus of chagasic megacolon after morphometric neuron analysis. Virchows Arch 438:254–258
Dias JC, Silveira AC, Schofield CJ (2002) The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz 97:603–612
Gattuso JM, Kamm MA (1993) Review article: The management of constipation in adults. Aliment Pharmacol Ther 7:487–500
Gama AH, Raia A, Netto AC (1971) Motility of the sigmoid colon and rectum: contribution to the physiopathology of megacolon in Chagas’ disease. Dis Colon Rectum 14:291–304
Okumura M, Netto AC (1963) [Etiopathogenesis of Chagas’ megacolon. Experimental contribution]. Rev Hosp Clin Fac Med Sao Paulo 18:351–360 (in Portuguese)
Grider JR (1989) Identification of neurotransmitters regulating intestinal peristaltic reflex in humans. Gastroenterology 97:1414–1419
Furness JB, Young HM, Pompolo S, Bornstein JC, Kunze WAA, McConalogue K (1995) Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology 108:554–563
Matini P, Faussone Pellegrini MS, Cortesini C, Mayer B (1995) Vasoactive intestinal polypeptide and nitric oxide synthase distribution in the enteric plexuses of the human colon: an histochemical study and quantitative analysis. Histochemistry 103:415–423
Wattchow DA, Furness JB, Costa M (1988) Distribution and coexistence of peptides in nerve fibers of the external muscle of the human gastrointestinal tract. Gastroenterology 95:32–41
Porter AJ, Wattchow DA, Brookes SJ, Schemann M, Costa M (1996) Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology 111:401–408
Koch TR, Carney JA, Go VL (1987) Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel diseases. Dig Dis Sci 32:369–376
Bernstein CN, Robert ME, Eysselein VE (1993) Rectal substance P concentrations are increased in ulcerative colitis but not in Crohn’s disease. Am J Gastroenterol 88:908–913
Mantyh CR, Vigna SR, Maggio JE, Mantyh PW, Bollinger RR, Pappas TN (1994) Substance P binding sites on intestinal lymphoid aggregates and blood vessels in inflammatory bowel disease correspond to authentic nk-1 receptors. Neurosci Lett 178:255–259
Holzer P (1998) Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion 59:269–283
Kishimoto S, Machino H, Kobayashi H, Haruma K, Kajiyama G, Miyoshi A, Fujii K (1994) Inhibitory action of cck-op on rat proximal colon. Ann NY Acad Sci 713:407–409
Kusafuka T, Puri P (1997) Altered mRNA expression of the neuronal nitric oxide synthase gene in Hirschsprung’s disease. J Pediatr Surg 32:1054–1058
Guo R, Nada O, Suita S, Taguchi T, Masumoto K (1997) The distribution and co-localization of nitric oxide synthase and vasoactive intestinal polypeptide in nerves of the colons with Hirschsprung’s disease. Virchows Arch 430:53–61
Vanderwinden JM, De Laet MH, Schiffmann SN, Mailleux P, Lowenstein CJ, Snyder SH, Vanderhaeghen JJ (1993) Nitric oxide synthase distribution in the enteric nervous system of Hirschsprung’s disease. Gastroenterology 105:969–973
Bealer JF, Natuzzi ES, Flake AW, Adzick NS, Harrison MR (1994) Effect of nitric oxide on the colonic smooth muscle of patients with Hirschsprung’s disease. J Pediatr Surg 29:1025–1029
Cuffari C, Rubin SZ, Krantis A (1993) Routine use of the nitric oxide stain in the differential diagnosis of Hirschsprung’s disease. J Pediatr Surg 28:1202–1204
O’Kelly T, Brading A, Mortensen N (1993) Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut 34:689–693
Burleigh DE, D’Mello A, Parks AG (1979) Responses of isolated human internal anal sphincter to drugs and electrical field stimulation. Gastroenterology 77:484–490
Gustafsson BI, Delbro DS (1993) Tonic inhibition of small intestinal motility by nitric oxide. J Auton Nerv Syst 44:179–187
Moore BG, Singaram C, Eckhoff DE, Gaumnitz EA, Starling JR (1996) Immunohistochemical evaluations of ultrashort–segment Hirschsprung’s disease. report of three cases. Dis Colon Rectum 39:817–822
Sanders KM, Ward SM, Thornbury KD, Dalziel HH, Westfall DP, Carl A (1992) Nitric oxide as a non-adrenergic, non-cholinergic neurotransmitter in the gastrointestinal tract. Jpn J Pharmacol 58(Suppl 2):220P–225P
O’Kelly TJ, Davies JR, Brading AF, Mortensen NJ (1994) Distribution of nitric oxide synthase containing neurons in the rectal myenteric plexus and anal canal. Morphologic evidence that nitric oxide mediates the rectoanal inhibitory reflex. Dis Colon Rectum 37:350–357
Li CG, Rand MJ (1989) Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol 16:933–938
Loder PB, Kamm MA, Nicholls RJ, Phillips RK (1994) ‘Reversible chemical sphincterotomy’ by local application of glyceryl trinitrate. Br J Surg 81:1386–1389
Lund JN, Scholefield JH (1997) A randomised, prospective, double-blind, placebo-controlled trial of glyceryl trinitrate ointment in treatment of anal fissure. Lancet 349:11–14
Schouten WR, Briel JW, Boerma MO, Auwerda JJ, Wilms EB, Graatsma BH (1996) Pathophysiological aspects and clinical outcome of intra-anal application of isosorbide dinitrate in patients with chronic anal fissure. Gut 39:465–469
Acknowledgments
This work was supported by funds from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Ministério da Educação, Brazil, and the National Health and Medical Research Council of Australia (Grant 400020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silveira, A.B.M., D’Avila Reis, D., de Oliveira, E.C. et al. Neurochemical Coding of the Enteric Nervous System in Chagasic Patients with Megacolon. Dig Dis Sci 52, 2877–2883 (2007). https://doi.org/10.1007/s10620-006-9680-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9680-5