Abstract
Background
Advanced fibrosis and cirrhosis (compensated advanced chronic liver disease [cACLD]) are clinically indistinguishable and increase risk of developing clinically significant portal hypertension. Baveno VII recommends using elastography to rule out and diagnose cACLD with liver stiffness measurement (LSM) cut-offs of 10/15 kPa.
Methods
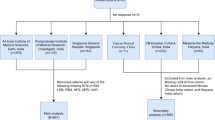
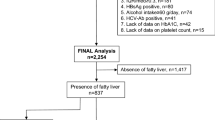
In a retrospective analysis of 330 nonalcoholic fatty liver disease (NAFLD) patients, performance of the Baveno VII cut-offs for diagnosing cACLD was compared with newly suggested lower cut-offs (8/12 kPa). A model for detecting cACLD among those with LSM between 8 and 12 kPa was developed and compared with recently published models.
Results
Seventy (21.2%) of the 330 NAFLD patients had biopsy-proven cACLD. The Baveno VII cut-offs (10/15 kPa) had a lower sensitivity of 72.8% (60.9–82.8%) and a specificity of 93.4% (89.7–96.1%). Sensitivity and specificity of lower cut-offs (8/12 kPa) were 91.4% (82.3–96.8%) and 88.5% (83.9–92.1%), respectively. Modeling based on the presence of diabetes (odds ratio [OR] 3.625[1.161–11.320], p = 0.027) and serum aspartate aminotransferase (AST) levels (OR 1.636[1.098–2.436], p = 0.015) correctly identified 75.7% of patients with LSM between 8 and 12 kPa. Our model performed best with an area under receiver operator curve (AUROC) of 0.725 (95%CI 0.609–0.822), compared to Papatheodoridi (AUROC 0.626, CI 0.506–.736) and Zhou (AUROC 0.523, CI 0.403–0.640) models. A two-step strategy comprising application of lower LSM cut-offs followed by the predictive model correctly identified the presence of cACLD in 83% of the patients as compared to 75% by the Baveno VII cut-offs.
Conclusion
A two-step strategy employing lower LSM cut-offs and modeling based on diabetes and AST levels outperforms Baveno VII cut-offs for identifying cACLD in NAFLD patients.




Similar content being viewed by others
Abbreviations
- cACLD:
-
Compensated advanced chronic liver disease
- LSM:
-
Liver stiffness measurement
- TE:
-
Transient elastography
- NAFLD:
-
Nonalcoholic fatty liver disease
- BMI:
-
Body mass index
- DM:
-
Diabetes mellitus
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- Alk P:
-
Alkaline phosphatase
- HBsAg:
-
Hepatitis B surface antigen
- FIB-4:
-
Fibrosis-4 index
- APRI:
-
AST-to-platelet ratio index
- CAP:
-
Controlled attenuation parameter
- IQR:
-
Interquartile range
- PPLB:
-
Percutaneous plugged liver biopsy
- NASH:
-
Nonalcoholic steatohepatitis
- CRN:
-
Clinical research network
- NAS:
-
NAFLD activity score
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- PLR:
-
Positive likelihood ratio
- NLR:
-
Negative likelihood ratio
- DA:
-
Diagnostic accuracy
- AUROC:
-
Area under receiver operator curve
- OR:
-
Odds ratio
- CSPH:
-
Clinically significant portal hypertension
- CPA:
-
Collagen proportionate area
- HVPG:
-
Hepatic venous pressure gradient
References
Augustin S, Pons M, Santos B, Ventura M, Genescà J. Identifying compensated advanced chronic liver disease: when (not) to start screening for varices and clinically significant portal hypertension. In: de Franchis R, ed. Berlin: Springer; 2016; 39–49. https://doi.org/10.1007/978-3-319-23018-4_5.
D’Amico G, Morabito A, D’Amico M et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–576. https://doi.org/10.1016/j.jhep.2017.10.020.
de Franchis R. Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. https://doi.org/10.1016/j.jhep.2015.05.022.
de Franchis R, Bosch J, Garcia-Tsao G et al. BAVENO VII - RENEWING CONSENSUS IN PORTAL HYPERTENSION: report of the Baveno VII Consensus Workshop: personalized care in portal hypertension. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.12.022.
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. American association for the study of liver diseases. Liver biopsy. Hepatology. 2009;49:1017–1044. https://doi.org/10.1002/hep.22742.
Zhou YJ, Gao F, Liu WY et al. Screening for compensated advanced chronic liver disease using refined Baveno VI elastography cutoffs in Asian patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:470–480. https://doi.org/10.1111/apt.16487.
Papatheodoridi M, Hiriart JB, Lupsor-Platon M et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol. 2021;74:1109–1116. https://doi.org/10.1016/j.jhep.2020.11.050.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. https://doi.org/10.1002/hep.28431.
Shalimar, Elhence A, Bansal B, et al. Prevalence of non-alcoholic fatty liver disease in India: A systematic review and meta-analysis. Journal of Clinical and Experimental Hepatology. Published online November 25, 2021. https://doi.org/10.1016/j.jceh.2021.11.010
Elhence A, Shalimar. Treatment of non-alcoholic fatty liver disease—current perspectives. Indian J Gastroenterol. 2020;39:22–31. https://doi.org/10.1007/s12664-020-01021-2.
Wong VWS, Irles M, Wong GLH et al. Unified interpretation of liver stiffness measurement by M and XL probes in non-alcoholic fatty liver disease. Gut. 2019;68:2057–2064. https://doi.org/10.1136/gutjnl-2018-317334.
Anand A, Elhence A, Vaishnav M et al. FibroScan-aspartate aminotransferase score in an Asian cohort of non-alcoholic fatty liver disease and its utility in predicting histological resolution with bariatric surgery. J Gastroenterol Hepatol. 2021;36:1309–1316. https://doi.org/10.1111/jgh.15358.
Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. https://doi.org/10.1002/hep.24127.
Kleiner DE, Brunt EM, Van Natta M et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. https://doi.org/10.1002/hep.20701.
Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264-1281.e4. https://doi.org/10.1053/j.gastro.2018.12.036.
Abraldes JG, Bureau C, Stefanescu H et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The “Anticipate” study. Hepatology. 2016;64:2173–2184. https://doi.org/10.1002/hep.28824.
Kumar R, Rastogi A, Sharma MK et al. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig Dis Sci. 2013;58:265–274. https://doi.org/10.1007/s10620-012-2306-1.
Eddowes PJ, Sasso M, Allison M et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–1730. https://doi.org/10.1053/j.gastro.2019.01.042.
Siddiqui MS, Vuppalanchi R, Van Natta ML et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156-163.e2. https://doi.org/10.1016/j.cgh.2018.04.043.
Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. Published online May 17, 2021:gutjnl-2021–324243. https://doi.org/10.1136/gutjnl-2021-324243
Lee SS. Radiologic evaluation of nonalcoholic fatty liver disease. WJG. 2014;20:7392. https://doi.org/10.3748/wjg.v20.i23.7392.
Ratziu V, Charlotte F, Heurtier A et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. https://doi.org/10.1053/j.gastro.2005.03.084.
Newsome PN, Sasso M, Deeks JJ et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. https://doi.org/10.1016/S2468-1253(19)30383-8.
Selvaraj EA, Mózes FE, Jayaswal ANA et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2021;75:770–785. https://doi.org/10.1016/j.jhep.2021.04.044.
Imajo K, Honda Y, Kobayashi T, et al. Direct comparison of US and MR elastography for staging liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. Published online December 17, 2020:S1542–3565(20)31673–6. https://doi.org/10.1016/j.cgh.2020.12.016
Acknowledgments
None
Funding
None to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Shalimar, Anshuman Elhence and Abhinav Anand. The first draft of the manuscript was written by Shalimar, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elhence, A., Anand, A., Biswas, S. et al. Compensated Advanced Chronic Liver Disease in Nonalcoholic Fatty Liver Disease: Two-Step Strategy is Better than Baveno Criteria. Dig Dis Sci 68, 1016–1025 (2023). https://doi.org/10.1007/s10620-022-07579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07579-5