Abstract
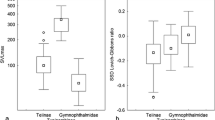
Rensch’s rule describes a pattern of allometry in sexual size dimorphism (SSD): when males are the larger sex (male-biased SSD), SSD increases with increasing body size, and when females are the larger sex (female-biased SSD), SSD decreases with increasing body size. While this expectation generally holds for taxa with male-biased or mixed SSD, examples of allometry for SSD consistent with Rensch’s rule in groups with primarily female-biased SSD are remarkably rare. Here, I show that the majority of dwarf chameleons (Bradypodion spp.) have female-biased SSD. In accordance with Rensch’s rule, the group exhibits an allometric slope of log(female size) on log(male size) less than one, although statistical significance is dependent on the phylogenetic comparative method used. In this system, this pattern is likely due to natural selection on both male and female body size, combined with fecundity selection on female body size. In addition to quantifying SSD and testing Rensch’s rule in dwarf chameleons, I discuss reasons why Rensch’s rule may only rarely apply to taxa with female-biased SSD.


Similar content being viewed by others
References
Abouheif E, Fairbairn DJ (1997) A comparative analysis of allometry for sexual size dimorphism: Assessing Rensch’s rule. Am Nat 149:540–562
Blanckenhorn WU, Dixon AFG, Fairbairn DJ, Foellmer MW, Gibert P, van der Linde K, Meier R, Nylin S, Pitnick S, Schoff C, Signorelli M, Teder T, Wiklund C (2007) Proximate causes of Rensch’s rule: does sexual size dimorphism in arthropods result from sex differences in development time? Am Nat 169:245–257
Branch WR (1998) A field guide to snakes and other reptiles of southern Africa 3rd edn. Struik Publishers, Cape Town
Burrage BR (1973) Comparative ecology and behaviour of Chamaeleo pumilis pumilis (Gmelin) and C. namaquensis A. Smith (Sauria: Chamaeleonidae). Ann S Afr Mus 61:1–158
Butler MA, Schoener TW, Losos JB (2000) The relationship between sexual size dimorphism and habitat use in Greater Antillean Anolis Lizards. Evolution 54:259–272
Colwell RK (2000) Rensch’s rule crosses the line: Convergent allometry of sexual size dimorphism in hummingbirds and flower mites. Am Nat 156:495–510
Diaz-Uriarte R, Garland T (1996) Testing hypotheses of correlated evolution using phylogenetically independent contrasts: Sensitivity to deviations from Brownian motion. Syst Biol 45:27–47
Fairbairn DJ (1997) Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu Rev Ecol Syst 28:659–687
Fairbairn DJ (2005) Allometry for sexual size dimorphism: testing two hypotheses for Rensch’s rule in the water strider Aquarius remigis. Am Nat 166:S69–S84
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Garland TJ, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32
Hansen TF (1997) Stabilizing selection and the comparative analysis of adaptation. Evolution 51:1341–1351
Jacobsen NHG (1989) A herpetological survey of the Transvaal. School of Life and Environmental Sciences, University of Natal, Durban
Jannot JE, Kerans BL (2003) Body size, sexual size dimorphism, and Rensch’s rule in adult hydropsychid caddisflies (Trichoptera : Hydropsychidae). Can J Zool 81:1956–1964
Kratochvil L, Frynta D (2002) Body size, male combat and the evolution of sexual dimorphism in eublepharid geckos (Squamata: Eublepharidae). Biol J Linn Soc 76:303–314
Martins EP (2004) COMPARE 4.6: statistical analysis of comparative data. pp. Available free from http://compare.bio.indiana.edu/. Dept. of Biology, Indiana University, Bloomington
Martins EP, Hansen TF (1997) Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149:646–667
Martins EP, Diniz JAF, Housworth EA (2002) Adaptive constraints and the phylogenetic comparative method: a computer simulation test. Evolution 56:1–13
Martins EP, Labra A, Halloy M, Thompson JT (2004) Large-scale patterns of signal evolution: an interspecific study of Liolaemus lizard headbob displays. Anim Behav 68:453–463
Nečas P (2001) Chameleons: Nature’s Hidden Jewels. Krieger Publishing, Malabar
Olsson M, Madsen T (1995) Female choice on male quantitative traits in lizards—why is it so rare? Behav Ecol Sociobiol 36:179–184
Raihani G, Szekely T, Serrano-Meneses MA, Pitra C, Goriup P (2006) The influence of sexual selection and male agility on sexual size dimorphism in bustards (Otididae). Anim Behav 71:833–838
Rensch B (1960) Evolution above the species level. Columbia University Press, New York
Shine R (1989) Ecological causes for the evolution of sexual size dimorphism. Quart Rev Biol 64:419–443
Stuart-Fox D, Moussalli A (2007) Sex-specific ecomorphological variation and the evolution of sexual dimorphism in dwarf chameleons (Bradypodion spp.). J Evol Biol 20:1073–1081
Stuart-Fox DM, Whiting MJ (2005) Male dwarf chameleons assess risk of courting large, aggressive females. Biol Lett 1:231–234
Stuart-Fox D, Moussalli A, Whiting MJ (2007) Natural selection on social signals: signal efficacy and the evolution of chameleon display coloration. Am Nat 170:916–930
Stuart-Fox DM, Firth D, Moussalli A, Whiting MJ (2006) Multiple traits in chameleon contests: designing and analysing animal contests as a tournament. Anim Behav 71:1263–1271
Szekely T, Freckleton RP, Reynolds JD (2004) Sexual selection explains Rensch’s rule of size dimorphism in shorebirds. Proc Natl Acad Sci USA 101:12224–12227
Tolley KA, Tilbury C, Branch WR, Matthee CA (2004) Phylogenetics of the southern African dwarf chameleons, Bradypodion (Squamata: Chamaeleonidae). Mol Phylogenet Evol 30:354–365
Tolley KA, Burger M, Turner AA, Matthee CA (2006) Biogeographic patterns and phylogeography of dwarf chameleons (Bradypodion) in an African biodiversity hotspot. Mol Ecol 15:781–793
Tubaro PL, Bertelli S (2003) Female-biased sexual size dimorphism in tinamous: a comparative test fails to support Rensch’s rule. Biol J Linn Soc 80:519–527
Vitousek MN, Mitchell MA, Woakes AJ, Niemack MD, Wikelski M (2007) High costs of female choice in a lekking lizard. PLoS One 2:e567
Young KA (2005) Life-history variation and allometry for sexual size dimorphism in Pacific salmon and trout. Proc R Soc Lond B 272:167–172
Zamudio KR (1998) The evolution of female-biased sexual size dimorphism: a population-level comparative study in horned lizards (Phrynosoma). Evolution 52:1821–1833
Acknowledgments
I am grateful to Adnan Moussalli for field assistance and critical comments on the manuscript and to Martin Whiting for facilitating this research. Funding was from a National Research Foundation (NRF) grant to DSF. Permits: MPB.5104 (Mpumalanga), 005-00001 (Limpopo), 1721/2003 and 4390/2005 (KZN), 234/2003 (Western Cape), WRO 11/03 WR (Eastern Cape).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stuart-Fox, D. A test of Rensch’s rule in dwarf chameleons (Bradypodion spp.), a group with female-biased sexual size dimorphism. Evol Ecol 23, 425–433 (2009). https://doi.org/10.1007/s10682-008-9242-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-008-9242-8