Abstract
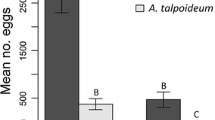
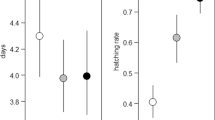
Population dynamics depends upon the spatial distribution of individuals in heterogeneous environments. The various processes surrounding insect oviposition are central to understanding their population dynamics because the choice of oviposition site ultimately influences the survivorship and spatial distribution of their progeny. Aquatic insects are often assumed to have non-selective oviposition habits, but empirical data are scarce and selective oviposition may be quite common. We quantitatively sampled egg masses of stream-dwelling caddisflies (Trichoptera) that specialise in egg-laying on hard substrata underwater, in order to characterise oviposition site selectivity and test for communal oviposition. In a field survey of two Scottish streams, we sampled egg masses of three species, Polycentropus flavomaculatus, Hydropsyche siltalai, Rhyacophila dorsalis, with the aim of testing whether egg mass abundance varied with current (riffles vs. pools), location within the channel (margins vs. centre) and rock exposure (emergent vs. fully submerged). In one stream, we captured adults landing on emergent rocks and assessed whether females were modified morphologically for swimming. The egg masses of two species (P. flavomaculatus, H. siltalai) occurred primarily on submerged rocks in pool margins, and adult females had legs modified for swimming. In contrast, egg masses of R. dorsalis were most abundant on the underside of emergent rocks in riffles, and females were not modified for swimming. Communal oviposition was evident for all three species, with most egg masses aggregated on the minority of potential rocks. How females locate oviposition sites and the consequences of these highly specialised oviposition behaviours to the survival and spatial distribution of larvae now require investigation. The effects of these behaviours on population dynamics are likely to differ from terrestrial herbivores because oviposition sites are not food resources for these aquatic species.




Similar content being viewed by others
References
Agosta, S. J., 2006. On ecological fitting, plant–insect associations, herbivore shifts, and host plant selection. Oikos 113: 556–656.
Awmack, C. S. & S. R. Leather, 2002. Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology 47: 817–844.
Badcock, R. M., 1953. Observation of oviposition under water of the aerial insect Hydropsyche angustipennis (Curtis) (Trichoptera). Hydrobiologia 5: 222–225.
Basaguren, A., P. Riano & J. Pozo, 2002. Life history patterns and dietary changes of several caddisfly (Trichoptera) species in a northern Spain stream. Archiv für Hydrobiologie 155: 23–41.
Blaustein, L. & B. P. Kotler, 1993. Oviposition habitat selection by the mosquito, Culiseta longiareolata: effects of conspecifics, food and green tadpoles. Ecological Entomology 18: 104–108.
Cohen, A. C. J., 1960. Estimating the parameter in a conditional Poisson distribution. Biometrics 16: 203–211.
Coupland, J. B., 1991. Oviposition response of Simulium reptans (Diptera: Simuliidae) to the presence of conspecific eggs. Ecological Entomology 16: 11–15.
Courtney, S. P., 1984. The evolution of egg clustering by butterflies and other insects. American Naturalist 123: 127–281.
Coutant, C. C., 1982. Positive phototaxis in first instar Hydropsyche cockerelli Banks (Trichoptera). Aquatic Insects 4: 55–59.
Crichton, M. I., D. Fisher & I. P. Woiwod, 1978. Life histories and distribution of British Trichoptera, excluding Limnephilidae and Hydroptilidae, based on Rothamsted Insect Survey. Holarctic Ecology 1: 31–45.
Davies, A., A. D. MacAdam & I. B. Cameron, 1986. Geology of the Dunbar District. Sheet 33E and part of Sheet 41 (Scotland). Memoir of the British Geological Survey, Scotland, UK.
Deutsch, W. G., 1984. Oviposition of Hydropsychidae (Trichoptera) in a large river. Canadian Journal of Zoology 62: 1988–1994.
Deutsch, W. G., 1985. Swimming modifications of adult female Hydropsychidae compared with other Trichoptera. Freshwater Invertebrate Biology 4: 35–40.
Doody, J. S., S. Freedberg & J. S. Keogh, 2009. Communal egg-laying in reptiles and amphibians: evolutionary patterns and hypotheses. Quarterly Review of Biology 84: 229–252.
Downes, B. J. & J. Jordan, 1993. Effects of stone topography on abundance of net-building caddisfly larvae and arthropod diversity in an upland stream. Hydrobiologia 252: 163–174.
Downes, B. J. & J. Lancaster, 2010. Does dispersal control population densities in advection-dominated systems? A fresh look at critical assumptions and a direct test. Journal of Animal Ecology 79: 235–248.
Edington, J. M. & A. G. Hildrew, 1995. Caseless caddis larvae of the British Isles. Scientific Publication No. 53, Freshwater Biological Association, Ambleside, UK.
Ellis, A. M., 2008a. Incorporating density dependence into the oviposition preference – offspring performance hypothesis. Journal of Animal Ecology 77: 247–256.
Ellis, A. M., 2008b. Linking movement and oviposition behaviour to spatial population distribution in the tree hole mosquito Ochlerotatus triseriatus. Journal of Animal Ecology 77: 156–166.
Encalada, A. C. & B. L. Peckarsky, 2006. Selective oviposition of the mayfly Baetis bicaudatus. Oecologia 148: 526–537.
Floater, G. J., 2001. Habitat complexity, spatial interference, and “minimum risk distribution”: a framework for population stability. Ecological Monographs 71: 447–468.
Fuller, R. L. & R. J. Mackay, 1980. Feeding ecology of three species of Hydropsyche (Trichoptera: Hydropsychidae) in southern Ontario. Canadian Journal of Zoology 58: 2239–2251.
Gibbons, D. W. & D. Pain, 1992. The influence of river flow rate on the breeding behaviour of Calopteryx damselflies. Journal of Animal Ecology 61: 281–289.
Gordon, N. D., T. A. McMahon, B. L. Finlayson, C. J. Gippel & R. J. Nathan, 2004. Stream Hydrology: An Introduction for Ecologists. John Wiley & Sons, Chichester, UK.
Hartley, S. & B. Shorrocks, 2002. A general framework for the aggregation model of coexistence. Journal of Animal Ecology 71: 651–662.
Hildrew, A. G. H. & R. Wagner, 1992. The briefly colonial life of hatchlings of the net-spinning caddisfly Plectrocnemia conspersa. Journal of the North American Benthological Society 11: 60–68.
Hinton, H. E., 1981. Biology of Insect Eggs, Vols. 1–3. Pergamon Press, Oxford.
Hoffmann, A. & V. H. Resh, 2003. Oviposition in three species of limnephilid caddisflies (Trichoptera): hierarchical influences in site selection. Freshwater Biology 48: 1064–1077.
Kuranishi, R. B., 1992. Oviposition behaviour of Rhyacophila kiyosumiensis Kuranishi (Trichoptera: Rhyacophilidae) in a small mountain stream, central Japan. In C. Otto (ed.), Proceedings of the Seventh International Symposium on Trichoptera. Backhuys, Leidan: 261–262.
Lancaster, J., B. J. Downes & P. Reich, 2003. Linking landscape patterns of resource distribution with models of aggregation in ovipositing stream insects. Journal of Animal Ecology 72: 969–978.
Lancaster, J., D. Bradley, A. Hogan & S. Waldron, 2005. Intraguild omnivory in predatory stream insects. Journal of Animal Ecology 74: 619–629.
Lancaster, J., B. J. Downes & A. Arnold, 2010. Environmental constraints on oviposition may limit density of a stream insect at multiple scales. Oecologia 163: 373–384.
Meadows, P. S. & J. I. Campbell, 1972. Habitat selection by aquatic invertebrates. Advances in Marine Biology 10: 271–382.
Muirhead-Thomson, R. C., 1956. Communal oviposition in Simulium damnosum Theobald (Diptera, Simuliidae). Nature 178: 1297–1299.
Muotka, T., 1990. Coexistence in a guild of filter feeding caddis larvae – do different instars act as different species. Oecologia 85: 281–292.
Osborne, L. L. & E. H. Herricks, 1987. Microhabitat characteristics of Hydropsyche (Trichoptera: Hydropsychidae) and the importance of body size. Journal of the North American Benthological Society 6: 115–124.
Petersen, R. C. J. & L. B.-M. Petersen, 1988. Compensatory mortality in aquatic populations: its importance for interpretation of toxicant effects. Ambio 17: 381–386.
Price, P. W., 1994. Phylogenetic constraints, adaptive syndromes, and emergent properties: from individuals to population dynamics. Researches on Population Ecology 36: 3–14.
Reich, P., 2004. Patterns of composition and abundance in macroinvertebrate egg masses from temperate Australian streams. Marine and Freshwater Research 55: 39–56.
Reich, P. & B. J. Downes, 2003a. Experimental evidence for physical cues involved in oviposition site selection of lotic hydrobiosid caddisflies. Oecologia 136: 465–475.
Reich, P. & B. J. Downes, 2003b. The distribution of aquatic invertebrate egg masses in relation to physical characteristics of oviposition sites at two Victorian upland streams. Freshwater Biology 48: 1497–1513.
Resetarits W. J. Jr., 1996. Oviposition site choice and life history evolution. American Zoologist 36: 205–215.
Rice, S. P., M. T. Greenwood & C. B. Joyce, 2001. Tributaries, sediment sources, and the longitudinal organisation of macroinvertebrate fauna along river systems. Canadian Journal of Fisheries and Aquatic Sciences 58: 824–840.
Rice, S. P., P. Kiffney, C. M. Greene & G. R. Pess, 2008. The ecological importance of tributaries and confluences. In Rice, S. P., A. G. Roy & B. L. Rhoads (eds), River Confluences, Tributaries and the Fluvial Network. John Wiley & Sons Ltd, Chichester, UK: 209–242.
Shorrocks, B. & J. G. Sevenster, 1995. Explaining local species diversity. Proceedings of the Royal Society of London B 260: 305–309.
Siva-Jothy, M. T., D. W. Gibbons & D. Pain, 1995. Female oviposition-site preference and egg hatching success in the damselfly Calopteryx splendens xanthosstoma. Behavioral Ecology and Sociobiology 37: 39–44.
Sokal, R. R. & F. J. Rohlf, 1981. Biometry. Freeman, New York.
Stamp, N. E., 1980. Egg deposition patterns in butterflies: why do some species cluster their eggs rather than deposit them singly? The American Naturalist 115: 367–380.
Stav, G., L. Blaustein & J. Margalit, 1999. Experimental evidence for predation risk sensitive oviposition by a mosquito, Culiseta longiareolata. Ecological Entomology 24: 202–207.
Swanson, C. A., 2004. Effect of substrate availability and conspecific cues on communal oviposition in the apple murex snail Phyllonotus pomum. Marine Ecology Progress Series 275: 175–184.
Thorson, G., 1964. Light as an ecological factor in the dispersal and settlement of marine bottom invertebrates. Ophelia 1: 167–208.
Willis, L. D. J. & A. C. Hendricks, 1992. Life history, growth, survivorship, and production of Hydropsyche slossonae in Mill Creek, Virginia. Journal of the North American Benthological Society 11: 290–303.
Acknowledgements
This project was supported by a grant awarded to JL and BJD by the Natural Environment Research Council, UK (NE/E004946/1). We are grateful to Beckie Langton, Isla McGregor and Rudi Verspoor for their sterling assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: D. Dudgeon
Rights and permissions
About this article
Cite this article
Lancaster, J., Downes, B.J. & Arnold, A. Oviposition site selectivity of some stream-dwelling caddisflies. Hydrobiologia 652, 165–178 (2010). https://doi.org/10.1007/s10750-010-0328-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0328-2