Abstract
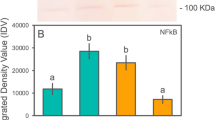
Recurrent disturbances on coral reefs that cause injuries, like predation and storm damage, and elevated seawater temperatures reduce coral fitness and immunocompetence. An effective immune response is essential to prevent disease and enhance colony survival. To evaluate how elevated seawater temperatures affect the coral immune response following injury, fragments of Acropora aspera were exposed to ambient (27–29°C) or elevated (32–33.5°C) seawater temperatures for 8 days and subsequently experimentally injured. Expression patterns for 15 immune genes 24 h post-injury revealed that most genes involved in the Toll-like receptor pathway were unaffected by elevated seawater temperatures. Exceptions to this pattern were cFos and cJun, which were upregulated and likely played a role in repair processes, and TRAF-6 and NFκB, which were downregulated suggesting reduced immune function. Components of the complement system were upregulated (millectin, C3) or downregulated (Bf, Tx60, apextrin) in corals at high temperatures. However, corals that also sustained injury, showed normal Tx60 and apextrin expression, suggesting roles in the wounding response. Overall, basal expression levels of immune genes are sufficient to mount a response to injury in the short term, and the immune response of A. aspera following injury is not significantly affected by minor elevations in seawater temperatures.




Similar content being viewed by others
References
Ainsworth, T. D., K. Wasmund, K. Wasmund, L. Ukani, F. Seneca, D. Yellowlees, D. Miller & W. Leggat, 2011. Defining the tipping point. A complex cellular life/death balance in corals in response to stress. Scientific Reports 1: 160.
Berkelmans, R. & B. L. Willis, 1999. Seasonal and local spatial variation in the upper thermal limits of corals in the central Great Barrier Reef. Coral Reefs 18: 219–228.
Bourne, D., Y. Iida, S. Uthicke & C. Smith-Keune, 2008. Changes in coral-associated microbial communities during a bleaching event. The ISME Journal 2: 350–363.
Burge, C. A., M. E. Mouchka, C. D. Harvell & S. Roberts, 2013. Immune response of the Caribbean sea fan, Gorgonia ventalina, exposed to an Aplanochytrium parasite as revealed by transcriptome sequencing. Frontiers in Physiology 4: 180.
Cerenius, L., S. Kawabata, B. L. Lee, M. Nonaka & K. Söderhäll, 2010. Proteolytic cascades and their involvement in invertebrate immunity. Trends in Biochemical Sciences 35: 575–583.
Cróquer, A., E. Villamizar & N. Noriega, 2002. Environmental factors affecting tissue regeneration of the reef—building coral Montastraea annularis (Faviidae) at Los Roques National Park, Venezuela. Revista de Biologia Tropical 50: 1055–1065.
Csaszar, N. B. M., F. O. Seneca & M. van Oppen, 2009. Variation in antioxidant gene expression in the scleractinian coral Acropora millepora under laboratory thermal stress. Marine Ecology Progress Series 392: 93–102.
Denis, V., M. M. M. Guillaumme, M. Goutx, S. de Palmas, J. Debreuil, A. C. Baker, R. K. Boonstra & J. H. Bruggemann, 2013. Fast growth may impair regeneration capacity in the branching coral Acropora muricata. PLoS One 8: e72618.
Edmunds, P. & H. Lenihan, 2010. Effect of sub-lethal damage to juvenile colonies of massive Porites spp. under contrasting regimes of temperature and water flow. Marine Biology 157: 887–897.
Erler, S., M. Popp & H. M. Lattorff, 2011. Dynamics of immune system gene expression upon bacterial challenge and wounding in a social insect (Bombus terrestris). Plos One 6: e18126.
Fehlbaum, P., P. Bulet, L. Michaut, M. Lagueux, W. F. Broekaert, C. Hetru & J. A. Hoffmann, 1994. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. The Journal of Biological Chemistry 269: 33159–33163.
Fine, M., U. Oren & Y. Loya, 2002. Bleaching effect on regeneration and resource translocation in the coral Oculina patagonica. Marine Ecology Progress Series 234: 119–125.
Franzenburg, S., S. Fraune, S. Kunzel, J. F. Baines, T. Domazet-Loso & T. C. G. Bosch, 2012. MyD88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers. Proceedings of the National Academy of Sciences of the United States of America 109: 19374–19379.
Franzenburg, S., J. Walter, S. Kunzal, J. Wang, J. F. Baines, T. C. G. Bosch & S. Fraune, 2013. Distinct antimicrobial peptide expression determines host species-specific bacterial associations. Proceedings of the National Academy of Sciences of the United States of America 110: E3730–E3738.
Fraune, S., R. Augustin & T. C. G. Bosch, 2011. Embryo protection in contemporary immunology: Why bacteria matter. Communicative & Integrative Biology 4: 369–372.
Fraune, S. & T. C. G. Bosch, 2007. Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra. Proceedings of the National Academy of Sciences of the United States of America 104: 13146–13151.
Grasso, L., J. Maindonald, S. Rudd, D. C. Hayward, R. Saint, D. J. Miller & E. E. Ball, 2008. Microarray analysis identifies candidate genes for key roles in coral development. BMC Genomics 9: 540.
Grasso, L. C., A. P. Negri, S. Foret, R. Saint, D. C. Hayward, E. E. Ball & D. J. Miller, 2011. The biology of coral metamorphosis: molecular responses of larvae to inducers of settlement and metamorphosis. Developmental Biology 353: 411–419.
Hamada, M., E. Shoguchi, C. Shinzato, T. Kawashima, D. J. Miller & N. Satoh, 2013. The complex NOD-like receptor repertoire of the coral Acropora digitifera includes novel domain combinations. Molecular Biology and Evolution 30: 167–176.
Henry, L.-A. & M. Hart, 2005. Regeneration from injury and resource allocation in sponges and corals—a review. International Review of Hydrobiology 90: 125–158.
Hess, J., P. Angel & M. Schorpp-Kistner, 2004. AP-1 subunits: quarrel and harmony among siblings. Journal of Cell Science 117: 5965–5973.
Hoegh-Guldberg, O., P. Mumby, A. Hooten, R. Steneck, P. Greenfield, E. Gomez, C. Harvell, P. Sale, A. Edwards & K. Cadeira, 2007. Coral reefs under rapid climate change and ocean acidification. Science 318: 1737–1742.
Lenihan, H. S. & P. J. Edmunds, 2010. Response of Pocillopora verrucosa to corallivory varies with environmental conditions. Marine Ecology Progress Series 409: 51–63.
Kimura, A., E. Sakaguchi & M. Nonaka, 2009. Multi-component complement system of Cnidaria: C3, Bf, and MASP genes expressed in the endodermal tissues of a sea anemone, Nematostella vectensis. Immunobiology 214: 165–178.
Kramarsky-Winter, E. & Y. Loya, 2000. Tissue regeneration in the coral Fungia granulosa: the effect of extrinsic and intrinsic factors. Marine Biology 137: 867–873.
Kvennefors, E. C. E., W. Leggat, O. Hoegh-Guldberg, B. M. Degnan & A. C. Barnes, 2008. An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts. Developmental and Comparative Immunology 32: 1582–1592.
Kvennefors, E. C. E., W. Leggat, C. C. Kerr, T. D. Ainsworth, O. Hoegh-Guldberg & A. C. Barnes, 2010. Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis. Developmental and Comparative Immunology 34: 1219–1229.
Lamb, J. B. & B. L. Willis, 2011. Using coral disease prevalence to assess the effects of concentrating tourism activities on offshore reefs in a tropical marine park. Conservation Biology 25: 1044–1052.
Lamb, J. B., J. D. True, S. Piromvaragorn & B. L. Willis, 2014. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biological Conservation 178: 88–96.
Leggat, W., F. Seneca, K. Wasmund, L. Ukani, D. Yellowlees & T. D. Ainsworth, 2011. Differential responses of the coral host and their algal symbiont to thermal stress. PLoS One 6: e26687.
Lindsay, S. M., 2010. Frequency of injury and the ecology of regeneration in marine benthic invertebrates. Integrative and Comparative Biology 50: 479–493.
Littman, R., B. L. Willis & D. G. Bourne, 2011. Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environmental Microbiology Reports 3: 651–660.
Mascarelli, P. E. & L. Bunkley-Williams, 1999. An experimental field evaluation of healing in damaged, unbleached and artificially bleached star coral, Montastraea annularis. Bulletin of Marine Science 65: 577–586.
Meesters, E. H., M. Pauchli & R. P. M. Bak, 1997. Predicting regeneration of physical damage on a reef-building coral by regeneration capacity and lesion shape. Marine Ecology Progress Series 146: 91–99.
Miller, D. J., G. Hemmrich, E. E. Ball, D. C. Hayward, K. Khalturin, N. Funayama, K. Agata & T. C. G. Bosch, 2007. The innate immune repertoire in Cnidaria—ancestral complexity and stochastic gene loss. Genome Biology 8: R59.
Mouchka, M. E., I. Hewson & C. D. Harvell, 2010. Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts. Integrative and Comparative Biology 50: 662–674.
Mydlarz, L. D., S. F. Holthouse, E. C. Peters & C. D. Harvell, 2008. Cellular responses in sea fan corals: granular amoebocytesreact to pathogen and climate stressors. Plos One 3: e1811.
Palmer, C. V., J. C. Bythell & B. L. Willis, 2011a. A comparative study of phenoloxidase activity in diseased and bleached colonies of the coral Acropora millepora. Developmental and Comparative Immunology 35: 1098–1101.
Palmer, C. V., N. G. Traylor-Knowles, B. L. Willis & J. C. Bythell, 2011b. Corals use similar immune cells and wound-healing processes as those of higher organisms. Plos One 6(8): e23992.
Palmer, C. V., J. C. Bythell & B. L. Willis, 2012. Enzyme activity demonstrates multiple pathways of innate immunity in Indo-Pacific anthozoans. Proceedings of the Royal Society B 279: 3879–3887.
Pollock, F. J., J. B. Lamb, S. N. Field, S. F. Heron, B. Schaffelke, G. Shedrawi, D. G. Bourne & B. L. Willis, 2014. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 9: e102498.
Pujol, N., S. Cypowyj, K. Ziegler, A. Millet, A. Astrain, A. Goncharov, Y. Jin, A. D. Chisholm & J. J. Ewbank, 2008. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Current Biology 18: 481–489.
Renegar, D. A., P. L. Blackwelder & A. L. Moulding, (2008). Coral ultrastructural response to elevated pCO2 and nutrients during tissue repair and regeneration. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida, 7–11 July 2008. 2: 1320–1324.
Ritchie, K. B., 2006. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Marine Ecology Progress Series 322: 1–14.
Rodriguez de la Vega, R. C., B. I. Garcia, C. D’Ambrosio, E. Diego-Garcia, A. Scaloni & L. D. Possani, 2004. Antimicrobial peptide induction in the haemolymph of the Mexican scorpion Centruroides limpidus limpidus in response to septic injury. Cellular and Molecular Life Sciences 61: 1507–1519.
Ruiz-Moreno, D., B. L. Willis, A. C. Page, E. Weill, A. Croquer, B. Vargas-Angel, A. G. Jordan-Garza, E. Jordan-Dahlgren, L. Raymundo & C. D. Harvell, 2012. Global coral disease prevalence associated with sea temperature anomalies and local factors. Diseases of Aquatic Organisms 100: 249–261.
Seneca, F. O., S. Foret, E. E. Ball, C. Smith-Keune, D. J. Miller & M. J. H. van Oppen, 2010. Patterns of gene expression in a scleractinian coral undergoing natural bleaching. Marine Biotechnology 12: 594–604.
Shinzato, C., E. Shoguchi, T. Kawashima, M. Hamada, K. Hisata, M. Tanaka, M. Fujie, M. Fujiwara, R. Koyanagi, T. Ikuta, A. Fujiyama, D. J. Miller & N. Satoh, 2011. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476: U320–U382.
Siboni, N., D. Abrego, F. O. Seneca, C. A. Motti, N. Andreakis, J. Tebben, L. L. Blackall & T. Harder, 2012. Using bacterial extract along with differential gene expression in Acropora millepora larvae to decouple the processes of attachment and metamorphosis. Plos One 7(5): e37774.
Sokolow, S., 2009. Effects of a changing climate on the dynamics of coral infectious disease: a review of the evidence. Diseases of Aquatic Organisms 87(1–2): 5–18.
Souter, P., L. K. Bay, N. Andreakis, N. Csaszar, F. O. Seneca & M. J. H. van Oppen, 2011. A multilocus, temperature stress-related gene expression profile assay in Acropora millepora, a dominant reef-building coral. Molecular Ecology Resources 11: 328–334.
Titlyanov, E. A. & T. V. Titlyanova, 2009. The dynamics of the restoration of mechanical damage to colonies of the scleractinian coral Porites lutea under conditions of competition with algal settlers for substratum. Russian Journal of Marine Biology 35: 230–235.
Titlyanov, E. A., T. V. Titlyanova, I. M. Yakovleva, Y. Nakano & R. Bhagooli, 2005. Regeneration of artificial injuries on scleractinian corals and coral/algal competition for newly formed substrate. Journal of Experimental Marine Biology and Ecology 323: 27–42.
Vandesompele, J., K. De Preter, F. Pattyn, B. Poppe, N. van Roy, A. de Paepe & F. Speleman, 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3: Research0034.
Vidal-Dupiol, J., O. Ladriere, D. Destoumieux-Garzon, P. E. Sautiere, A. L. Meistertzheim, E. Tambutte, S. Tambutte, D. Duval, L. Foure, M. Adjeroud & G. Mitta, 2011a. Innate immune responses of a scleractinian coral to vibriosis. Journal of Biological Chemistry 286: 22688–22698.
Vidal-Dupiol, J., O. Ladriere, A. L. Meistertzheim, L. Foure, M. Adjeroud & G. Mitta, 2011b. Physiological responses of the scleractinian coral Pocillopora damicornis to bacterial stress from Vibrio coralliilyticus. Journal of Experimental Biology 214: 1533–1545.
Vidal-Dupiol, J., N. M. Dheilly, R. Rondon, C. Grunau, C. Cosseau, K. M. Smith, M. Freitag, M. Adjeroud & G. Mitta, 2014. Thermal stress triggers broad Pocillopora damicornis transcriptomic remodeling, while Vibrio coralliilyticus infection induces a more targeted immuno-suppression response. PLoS One 9(9): e107672.
Willis, B. L., C. A. Page & E. A. Dinsdale, 2004. Coral disease on the Great Barrier Reef. In Rosenberg, E. & Y. Loya (eds), Coral Health and Disease. Springer, Berlin: 69–104.
Witt, V., C. Wild, K. R. Anthony, G. Diaz-Pulido & S. Uthicke, 2011. Effects of ocean acidification on microbial community composition of, and oxygen fluxes through, biofilms from the Great Barrier Reef. Environmental Microbiology 13: 2976–2989.
Wu, X. & G. Brewer, 2012. The regulation of mRNA stability in mammalian cells: 2.0. Gene 500: 10–21.
Xu, Q., G. Wang, H. Yuan, Y. Chai & Z. Xiao, 2010. cDNA sequence and expression analysis of an antimicrobial peptide, theromacin, in the triangle-shell pearl mussel Hyriopsis cumingii. Comparative Biochemistry and Physiology Part B 157: 119–126.
Acknowledgments
The authors would like to thank the Australian Research Council for the funding provided.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Yehuda Benayahu, Oren Levy & Tamar Lotan / Coelenterate Biology: Advanced Studies on Cnidaria and Ctenophora
Rights and permissions
About this article
Cite this article
van de Water, J.A.J.M., Leggat, W., Bourne, D.G. et al. Elevated seawater temperatures have a limited impact on the coral immune response following physical damage. Hydrobiologia 759, 201–214 (2015). https://doi.org/10.1007/s10750-015-2243-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2243-z