Abstract
Pelagic larvae of benthic organisms comprise a substantial part of the coastal Arctic zooplankton community in spring–summer. We studied the timing, growth, and pelagic duration of Cirripedia larvae in Adventfjorden, a high-Arctic fjord in Spitsbergen, Svalbard. Two distinct abundance peaks were found: one in early May (~ 25.000 ind. m−3) and another one in late May (~ 35,580 ind m−3). DNA barcoding based on the COI gene was used to identify the barnacle larvae to species. Whereas both Balanus balanus and Semibalanus balanoides were present, the first one dominated (50–100%) the barnacle abundance. High resolution sampling and size measurements of Cirripedia larvae revealed that these larvae most likely originated from a single spawning event. Development of the larvae suggested a pelagic residence time of roughly 2 months for B. balanus and at least 1 month for S. balanoides in the Arctic. Long pelagic residence time, large potential for biofouling on ships and larger plastic debris, combined with the disappearance of landfast sea ice and less ice scouring opens up new opportunities for barnacles to colonize the high-Arctic littoral zone. In a future warmer Arctic, we therefore expect establishment of new, more temperate Cirripedia species in Svalbard.
Similar content being viewed by others
Introduction
Marine sessile benthic invertebrates often have a pelagic larvae phase in their life cycle to be able to spread to new areas (Mileikovsky, 1971). Marine invertebrates produce high number of eggs, reaching from several thousands to several millions, however only a small fraction of which will survive and go through metamorphosis and finally settle at the bottom (Thorson, 1950). Planktonic development is crucial for survivorship of benthic sessile populations, as larvae may colonize new territories and thus, reduce intra-specific food competition. These larvae may temporarily comprise a significant part of the zooplankton community. Meroplankton can surpass holoplankton not only in terms of abundance, but also in terms of biomass (Clough et al., 1997; Stübner et al., 2016) and thus likely play an important ecological role both as grazers and as prey for organisms such as carnivorous zooplankton or numerous fish larvae (Coyle & Paul, 1990).
Barnacles are sessile crustaceans, living on hard substrata or on other organisms. They inhabit rocky bottom and man-made constructions, where they often dominate in percentage coverage and in terms of benthic community biomass (Hop et al., 2002). In the Svalbard Archipelago, only three species are reported in shallow waters: the arctic-boreal Balanus balanus (Linnaeus, 1758), the boreo-arctic Semibalanus balanoides (Linnaeus, 1767), and in a few occasions the boreal Balanus crenatus (Bruguière, 1789) (Anisimova et al., 2010). These barnacles are hermaphrodites. B. balanus produce one single brood per year and the nauplii are liberated into the water after ca 40 days of embryonic development (Barnes & Barnes, 1954). How many nauplii B. balanus can produce is dependent on the size of the adult and varies between 3,000 and 100,000 individuals (Barnes & Barnes, 1954). The release of Cirripedia larvae is timed to the spring bloom and > 30,000 individuals per m3 can be found in Svalbard fjords when algal food is plentiful (Kuklinski et al., 2013; Stübner et al., 2016).
The first morphological descriptions of barnacle larvae were created in the 1940s (Pyefinch, 1948), and they have been developed since (e.g.: Crisp, 1962; Walley, 1968). Cirripedia larvae undergo six naupliar developmental stages and finally metamorphose into a non-feeding cyprid larvae stage, which is ready for settling (Bassindale, 1936). Even though Cirripedia constitute a numerically important part of the zooplankton community, their species-specific timing and pelagic duration is poorly known.
Balanus balanus and S. balanoides are abundant in shallow waters down to 60 m depth (Barnes & Barnes, 1954). Reproduction of these species is directly related to temperature and food abundance. According to existing knowledge, breeding of these barnacles will not occur at temperature above 10°C (Barnes & Barnes, 1954).
The first report of S. balanoides in Svalbard waters (under its former name Balanus balanoides) is from 1953 (Feyling-Hanssen, 1953). Even though the species was found in different fjords, its reproduction was not successful in all years due to unfavorable cold environmental conditions and the presence of sea ice (Feyling-Hanssen, 1953). The population of S. balanoides located near Longyearbyen (Isfjorden, Svalbard) at that time was suggested to be unstable, probably due to unsuitable substratum (Feyling-Hanssen, 1953).
The recent warming of Arctic seas enables penetration of boreal species into Arctic waters (Berge et al., 2005; Kraft et al., 2013). The opening up of new habitat is particularly relevant to Cirripedia, as their larvae may be transported northwards by the ballast waters of ships (Ware et al., 2016).
Studies on meroplankton have been limited since most benthic invertebrate larvae cannot easily be identified to the species based on morphology. Furthermore, the invertebrates go through metamorphosis and often possess several larval developmental stages, thus determination of a particular stage may be challenging (Pradillon et al., 2007). Molecular tools based on DNA barcoding (Bucklin et al., 2011) may provide a quicker and easier approach to species determination of e.g., meroplankton. This technique has been successfully applied for meroplankton identification in the Antarctic (Heimeier et al., 2010), and in the Arctic for Bivalvia larvae (Brandner et al., 2017).
In this study, we used DNA barcoding to investigate species diversity among Cirripedia larvae in a high-Arctic fjord. An earlier study of meroplankton abundances in Adventfjorden, West Spitsbergen identified two abundance peaks of Cirripedia during their 2012 year-round study: one in early May at the onset of the phytoplankton bloom and another one in the end of May (Stübner et al., 2016). In this study, we used material collected by Stübner et al. (2016) and aimed to test the hypothesis that the two peaks constituted separate Cirripedia species. To test this hypothesis, we optimized the DNA extraction method to obtain good quality DNA from limited amount of material (i.e., a singular Cirripedia larva). We used the mitochondrial COI (cytochrome c oxidase I) gene to identify the barnacle larvae to species. Furthermore, we measured the Cirripedia larval sizes to be able to follow their development and timing of release, as well as their growth to estimate their pelagic residence time in a high-Arctic fjord.
Materials and methods
Study area
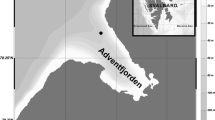
Samples were collected at the IsA (Isfjorden, Adventfjorden) time series station (78.261N 15.535E) situated at the entrance of Adventfjorden, a side branch of Isfjorden (Fig. 1), the largest fjord on West Spitsbergen (Svalbard Archipelago). This fjord system has since 2006 been strongly influenced by the West Spitsbergen Current, which provides warm and salty waters of Atlantic origin (Nilsen et al., 2008, 2016), and as a result Adventfjorden has during the last decade had a sub-Arctic character with no seasonal sea ice cover (Wiedmann et al., 2016).
Sampling
Zooplankton samples were taken by a WP2 net with 63 µm mesh size and opening area of 0.25 m2. Environmental data, like temperature and salinity, were collected by a hand-held CTD (SAIV SD204) and chlorophyll a was measured fluorometrically from water samples as described by Stübner et al. (2016). Samples were collected monthly throughout the entire year 2012 with up to weekly resolution during the peak primary production season (Stübner et al., 2016). For the purpose of this study we analyzed Cirripedia larvae from seven dates (Table 1) from the upper water layer (25–0 m) over a 10 weeks period from late April (27.04.2012) to July (06.07.2012), in which Cirripedia larvae were present in the pelagic according to Stübner et al. (2016). Stübner et al. (2016) analyzed the 4% formalin preserved samples in their study, while we used the parallel sample set preserved in 96% ethanol for molecular analyses. These ethanol samples were stored at 4°C until DNA extraction in 2017.
Molecular analyses
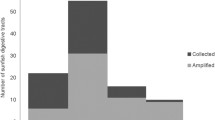
In total 243 larvae were removed from the 7 ethanol samples collected in the period from April to July. A subsample of 2 ml was taken and larvae were measured and photographed by a Leica M125C stereo microscope before they were rehydrated for 2 h in distilled water prior to molecular analyses.
DNA extraction
Commercial kits for DNA extraction like the DNeasy Blood & Tissue Kit (Qiagen) or the Tissue DNA Kit (Omega) were tested, but limited or no DNA remained from the larvae based on kit extractions. We also tested the method of Heimeier et al. (2010), who used 10 mg ml−1 of proteinase K and 5% chelex diluted in water, but the DNA yield was not satisfactory based on that protocol either. Subsequently, different combinations of proteinase K and chelex concentrations, volumes of the solution, as well as different incubation temperatures were tested. It turned out that the DNA extraction based only on chelex gave the best results thus proteinase K was excluded from further experiments. Finally, DNA extraction of individual larva was done using the following protocol: single larva was transferred to Eppendorf tubes and incubated in 30 µl of 10% chelex in TE buffer for 20 min in 97°C. The samples were then centrifuged at 14,000 rpm for 5 min and the supernatant, containing the extracted DNA, was transferred to a new Eppendorf tube. The extracted DNA was used directly as a template in PCR (Polymerase Chain Reaction).
DNA amplification
Larval DNA was amplified with Metazoa mitochondrial primers (Folmer et al., 1994) targeting a 600 bp fragment of COI (cytochrome oxidase subunit I). PCR amplifications of the COI fragment were performed in 20 μl volumes with 1 μl template DNA, 0.25 U of DreamTaq polymerase, 1 X Taq buffer, 0.2 μM of each primer, 0.25 μM of each dNTP, and 0.5 mg/ml of BSA using the following conditions: initial denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 48°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 3 min. Quality control of PCR products was undertaken by a gel electrophoresis in a 1% agarose gel at 210 V for 10 min. DNA was purified using solid-phase reversible immobilization (SPRI). Positive PCR products (totally 168; Table 1) were Sanger sequenced in one direction at GATC Biotech (European Genome and Diagnostics Centre), Germany.
Cirripedia size measurements
From the formalin preserved samples from 27th April to 6th July 2012, minimum 30 Cirripedia larvae (nauplii and cyprids) were counted and measured from a known volume (subsamples of 1 ml were taken by a wide mouthed pipette) per sampling date. The surface 0–25 m samples were studied, and in July also the bottom layer from 25 to 65 m depth since no Cirripedia nauplii and very few cyprids were found in the 0–25 m sample layer. The individual carapace lengths of nauplii and cyprids were measured according to West & Costlow (1987) to nearest 1.0 µm under a Leica MZ12 stereomicroscope with 32× or 40× magnification.
Data analysis
Sequences were quality checked, aligned, and analyzed in Geneious 10.1.3 (http://www.geneious.com; Kearse et al., 2012). Sequence distances were calculated in MEGA7 based on K2P with 1,000 bootstrap replications in order to obtain the highest possible credibility and lower bias in obtained results (Kumar et al., 2016), then percentage of differences in nucleotide positions within and between species was calculated manually.
Cirripedia abundances and sizes were plotted in R Studio (RStudio Team, 2015) with ggplot2 (Wickham, 2016).
Results
Cirripedia abundances
At the IsA station in Adventfjorden zooplankton samples were collected throughout the entire year in 2012 (Stübner et al., 2016), but Cirripedia nauplii were only found during the period from 27th April to 6th July with the exception of 1–2 occurrences outside this 10-week time period (Fig. 2). The abundance of nauplii was overall much higher in the upper 25 m, whilst cyprids were more numerous in the lower layer (Fig. 2). Two distinct peaks in abundance were observed during the peak chlorophyll a time period; one in early May (35,580.5 ind m−3) and another one (36,820.4 ind m−3) 3 weeks later at the end of May (Fig. 2). In July, nauplii had vanished, but cyprids were still around and mainly in the lower layer of the water column (bottom depth 80 m).
Cirripedia species
In total 168 Cirripedia COI sequences of 600 bp were obtained from the period 27th April to 6th July, and the amplification success was on average 68% (range 40 to 90%, Table 1). Based on BLAST analysis (similarity > 99%) Cirripedia larvae were assigned into two species: B. balanus (n = 156) and S. balanoides (n = 12). We detected genetic distance values of: B. balanus 0.009, with standard error 0.001 and S. balanoides 0.01 with error 0.003. The genetic distance between species was 0.162 with standard deviation 0.015. The nucleotide divergence between sequences of B. balanus and S. balanoides was 15%, while variation within species was 2.4% for B. balanus and 0.9% for S. balanoides, respectively. Hence, the barcoding gap between these two species was distinct.
Species-environment relationships
In April and May, when sea temperatures were low (− 0.3 to 0.6°C), and chlorophyll a concentrations high (max 4.4 µg Chl a l−1), only nauplii of Cirripedia were present (Fig. 3). These nauplii comprised of two species, but mainly of B. balanus (Table 1, Fig. 3). S. balanoides was rare (< 10%), except at the end of May when it comprised around 30% of all Cirripedia nauplii. In June–July, Cirripedia were mainly present as cyprids and only the species B. balanus were confirmed present by molecularly analyses of both nauplii and cyprids (Table 1, Fig. 3). In July, when the chlorophyll a biomass was very low (< 1 µg Chl a l−1) only cyprids of B. balanus were found.
Cirripedia larvae sizes
The length of the Cirripedia nauplii varied from 380 µm to 870 µm, while cyprids varied from 740 to 1,070 µm in size (Fig. 4). In late April only nauplii of smaller sizes were present (Fig. 4). Nauplii consistently increased in size with time and at 14 June cyprids were for the first time found in relatively high numbers (Fig. 4).
Discussion
Our working hypothesis was rejected, as the two abundance peaks of Cirripedia (3rd and 30th of May) were not related to different species. Both peaks comprised the two species B. balanus and S. balanoides, but with B. balanus dominating in terms of abundance in both the first (> 92%) and second (68%) peak. B. balanus and S. balanoides have similar naupliar sizes and can thus not be determined to species based on size alone (Crisp, 1962). Nauplii sizes consistently increased with time and the nauplii sizes on the 30th May were distinctly larger than those of 3rd May. This strongly suggests that the second peak was not due to a second massive nauplii release of S. balanoides. From late April to July, local water masses dominated in Adventfjorden and the entire water column was well mixed until a warmer, surface freshwater layer appeared in June (Stübner et al., 2016). Advection was thus minor and the second Cirripedia peak was likely rather a coincidence of patchiness potentially caused locally by tidal currents combined with movement of the upper surface layer due to for instance wind (e.g., Michelsen et al., 2017).
Barnacles time their nauplii release to optimal feeding conditions (Arendt et al., 2013). They keep the ready nauplii in their body cavity and release them at the onset of the spring bloom (Barnes, 1962). Cirripedia nauplii are therefore regarded as valid spring-bloom indicators (Arendt et al., 2013; Stübner et al., 2016; Michelsen et al., 2017). In this study, B. balanus released their nauplii in April when the chlorophyll a biomass increased, while for S. balanoides the nauplii release is difficult to estimate since their overall abundance was very low compared to B. balanus and thus may simply have been overlooked in April. S. balanoides was only confirmed present in May and no cyprids were confirmed to be S. balanoides. At the end of May only a few cyprids were found and these were not identified to species by molecular tools. The first sampling in June was on the 14th so cyprids of S. balanoides may have been present and already settled during this 14-days period.
Cirripedia in the northern hemisphere may be rather long-lived and might not reproduce every year. Lack of reproduction occurs rather rarely, for instance when there is a long-lasting sea ice cover combined with a severe delay in the spring bloom (Feyling-Hanssen, 1953). However, temperature-dependent variation in recruitment has been shown to cause strong year to year fluctuations in recruitment of S. balanoides (Rognstad & Hilbish, 2014).
According to Silberberger et al. (2016), there is a temporal succession of barnacle larvae due to food availability. Experiments have shown that particular barnacle species have different preferences, for instance, S. balanoides feed on diatoms (Stone, 1989). As a result, S. balanoides may appear in the water column at slightly different times than B. balanus. We have to treat our data with caution since we only sampled one site (e.g., Michelsen et al., 2017), but in our study, S. balanoides seemed to narrow their pelagic presence to the peak spring bloom in May when diatoms were abundant (Kubiszyn et al., 2017).
High resolution sampling allowed us to follow the barnacles’ development or more precise growth in size. At the end of April, only smaller naupliar sizes were present with a continuous size increase in the following sampling dates until mid-June when the Cirripedia larvae started to go through metamorphosis from naupliar to cypris stage. Crisp (1962) described the development of B. balanus and S. balanoides (former B. balanoides) from the British waters. He found a continuous increase in naupliar size with latitude for S. balanoides but not for B. balanus. The naupliar size ranges observed in our study fit well with those reported by Crisp (1962) for B. balanus in the Arctic, and less for S. balanoides which may be up to 1.6 mm long in the Arctic. Despite his very detailed naupliar studies, Crisp (1962) did not estimate the pelagic residence time for Cirripedia larvae. Based on the obtained data, and analysis of size spectra we estimated the pelagic residence time to be around 8 weeks in total (6 weeks of naupliar stage and 1 to 2 weeks of cypris) for B. balanus. These findings are in the agreement with data obtained for B. balanus from the Rhode Island, east coast of United States waters, for which the plankton stage lasted 45 days (Lang & Ackenhusen-Johns, 1981). From all studies for which data on Cirripedia larval duration are available, the most similar research area to ours is the Avacha Inlet in Kamchatka, where larvae of B. balanus were present for around 2 months (Korn & Kulikova, 1995). For S. balanoides the pelagic residence time depends more on food availability than the temperature and is estimated to be between 30 and 40 days (Barnes & Barnes, 1976), so 2–3 weeks shorter than for B. balanus which fits nicely with our data.
An increase in sea temperature and decrease in the winter sea ice cover has been observed in Isfjorden the last decade (Muckenhuber et al., 2016), explaining why species of boreal-Arctic and boreal origin commonly appear in Isfjorden (Gluchowska et al., 2016). This finding is not so surprising, taking into an account, its sub-Arctic sea temperatures, despite being as far North as 78°N. In fact, the mild conditions of the ice-free sea allow the colonization of temperate species such as the blue mussel Mytilus edulis (Berge et al., 2005). Changes in environmental conditions, like increasing temperature may modify the timing of barnacle reproduction, as it has been seen for other invertebrate species in the same area (Weydmann et al., 2018). As a result, populations of the boreo-Arctic S. balanoides are well established in Svalbard waters and this species is expected to increase its range even further North and East in the future. Other, even more boreal Cirripedia species like B. crenatus and Amphibalanus improvisus may also likely commonly occur in Svalbard in the years to come. A. improvisus has already been found on ships in Arctic harbors (Chan et al., 2016). Barnacles, as organisms possessing planktonic larvae and wide tolerance for environmental conditions, have a high ability for spreading into new territories. In 2016, Ware et al. (2016) reported the observation of A. improvisus larvae in ballast water of ships in Svalbard and according to his analyses, the current environment in the Arctic is suitable for colonization of non-native barnacle species. Furthermore, our research has shown that larval development in barnacles is long, hence larvae can travel long distances in ballast waters. In our study, non-native species were not found; nevertheless, such possibility should be addressed in future studies. Fast detection of invasive species is not an easy task, and that is why we recommend permanent monitoring, using molecular methods as presented in our study to be able to detect early sign of new colonizers.
DNA extraction from individual larvae required some optimization (see Materials and Methods and references therein), but our PCR success rate of almost 70% is fairly good compared to similar studies from Antarctic waters, e.g., Webb et al. (2006) with 22% success rate and Heimeier et al. (2010) with 35% success rate. Thus, our optimized extraction method for Cirripedia larvae may work well also in other studies and potentially for other larval groups. Another approach that could be used is metabarcoding or eDNA. eDNA allows for extraction of DNA from an environmental sample: if Cirripedia were present in the water, their DNA should be present in the sample material retained on the filter after water filtration. In metabarcoding, various samples can be pooled together, thus laboratory analysis may be more efficient, as extraction of DNA from individual larvae is not necessary (Walczyńska et al., 2018). Both approaches involve Next Generation Sequencing methods and have many advantages including detection of rare and less abundant species, however, they provide only qualitative species data. Currently, there is no way to obtain quantitative abundance data, as number of sequences is related to copy number of a particular gene, not number of organisms (Bucklin et al., 2016).
In conclusion, the appearance of Cirripedia larvae in the water column was strongly related to the algal food availability. In Isfjorden two species of Cirripedia were found, which co-occurred for parts (i.e., in May) of the period from April to July when Cirripedia were present in the water column. Two peaks of Cirripedia abundance (early and late May) were found and both of them were dominated by the same species: B. balanus, with rather small contribution of S. balanoides. Our study has shown that Cirripedia have long pelagic residence time (up to 2 months). This long pelagic presence combined with warmer sea temperatures, disappearance of landfast sea ice and thus less ice scouring opens up new opportunities for barnacles to colonize the high-Arctic littoral zone. In the coming years, we expect to find an increase in barnacles in the ice scouring zone as well as establishment of new, more temperate Cirripedia species in Svalbard.
References
Anisimova, N. A., L. L. Jørgensen, P. A. Lyubin & I. E. Manushin, 2010. Mapping and monitoring of benthos in the Barent Sea and Svalbard waters: Results from the joint Russian–Norwegian benthic programme 2006–2008. IMR–PINRO Joint Report Series 1–2010. ISSN 1502–8828. 114.
Arendt, K. E., T. Juul-Pedersen, J. Mortensen, M. E. Blicher & S. Rysgaard, 2013. A 5-year study of seasonal patterns in mesozooplankton community structure in a sub-Arctic fjord reveals dominance of Microsetella norvegica (Crustacea, Copepoda). Journal of Plankton Research 35: 105–120.
Barnes, H., 1962. Note on the variations in the release of nauplii of Balanus balanoides with special reference to the spring diatom outburst. Crustaceana 4: 118–122.
Barnes, H. & M. Barnes, 1954. The general biology of Balanus balanus (L.) Da Costa. Oikos 5: 63–76.
Barnes, H. & M. Barnes, 1976. The rate of development of the embryos of Balanus balanoides (L.) from a number of European and American populations and the designation of local races. Journal of Experimental Marine Biology and Ecology 24: 251–269.
Bassindale, R., 1936. The developmental stages of three English barnacles, Balanus balanoides (Linn.), Chlhamalas stellatas (Poli) and Verruca stroemia (OF Müller). Journal of Zoology 106: 57–74.
Berge, J., G. Johnesn, F. Nilsen, B. Gulliksen & D. Slagstad, 2005. Ocean temperature oscillations enable reapperance of blue mussels Mytilus edulis in Svalbard after a 1000 year absence. Marine Ecology Progress Series 303: 167–175.
Brandner, M. M., E. Stübner, A. J. Reed, T. M. Gabrielsen & S. Thatje, 2017. Seasonality of bivalve larvae in a high Arctic fjord. Polar Biology 40: 236. https://doi.org/10.1007/s00300-016-1950-x.
Bucklin, A., D. Steinke & L. Blanco-Bercial, 2011. DNA barcoding of marine metazoan. Annual Review of Marine Science 3: 471–508.
Bucklin, A., P. K. Lindeque, N. Rodriguez-Ezpeleta, A. Albaina & M. Lehtiniemi, 2016. Metabarcoding of marine zooplankton: prospects, progress and pitfalls. Journal of Plankton Research 38: 393–400.
Chan, F. T., H. J. MacIsaac & S. A. Bailey, 2016. Survival of ship biofouling assemblages during and after voyages to the Canadian Arctic. Marine Biology 163: 250.
Clough, L. M., W. G. Ambrose Jr., C. J. Ashjian, D. Piepenburg, P. E. Renaud & S. L. Smith, 1997. Meroplankton abundance in the Northeast Water Polynya: insights from oceanographic parameters and benthic abundance patterns. Journal of Marine Systems 10: 343–357.
Coyle, K. O. & A. J. Paul, 1990. Abundance and biomass of meroplankton during the spring bloom in an Alaskan Bay. Ophelia 32: 199–210.
Crisp, D. J., 1962. The Planktonic Stages of Cirripedia Balanus balanoides (L.) and Balanus balanus (L.) from North Temperate Waters. Marine Biology Station, Menai Bridge: 207–221.
Feyling-Hanssen, R. W., 1953. The Barnacle Balanus balanoides (Linné, 1766) in Spitsbergen. Norsk Polarinstitutt, Skrifter 98: 1–80.
Folmer, O., M. Black, W. Hoeh, R. Lutz & R. Vrijenhoeck, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Gluchowska, M., S. Kwasniewski, A. Prominska, A. Olszewska, I. Goszczko, S. Falk-Petersen, H. Hop & J. M. Weslawski, 2016. Zooplankton in Svalbard fjords on the Atlantic-Arctic boundary. Polar Biology 39: 1785–1802.
Heimeier, D., S. Lavery & M. A. Sewell, 2010. Using DNA barcoding and phylogenetics to identify Antarctic invertebrate larvae: lesson from a large scale study. Marine Genomics 3: 165–177.
Hop, H., T. Pearson, E. N. Hegseth, K. M. Kovacs, C. Wienckle, S. Kwasniewski, K. Eiane, F. Mehlum, B. Gulliksen, M. Wlodarska-Kowalczuk, C. Lydersen, J. M. Weslawski, S. Cochrane, G. W. Gabrielsen, R. J. G. Leakey, O. J. Lønne, M. Zajaczkowski, S. Falk-Petersen, M. Kendall, S.-A. Wängberg, K. Bischof, A. Y. Voronkov, N. A. Kovaltchouk, J. Wiktor, M. Poltermann, G. di Prisco, C. P. Gerland & S. Gerland, 2002. The marine ecosystem of Kongsfjorden, Svalbard. Polar Research 21: 167–208.
Kearse, M., R. Moir, A. Wilson, S. Stones-Havas, M. Cheung, S. Sturrock, S. Buxton, A. Cooper, S. Markowitz, C. Duran, T. Thierer, B. Ashton, P. Mentjies & A. Drummond, 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649.
Korn, O. M. & V. A. Kulikova, 1995. Seasonal species distribution of barnacle larvae in Avacha Inlet (Kamchatka). Journal of Plankton Research 17: 221–234.
Kraft, A., E. M. Nöthig, E. Bauerfeind, D. J. Wildish, G. W. Pohle, U. V. Bathmann, A. Beszczynska-Möller & M. Klages, 2013. First evidence of reproductive success in a southern invader indicates possible community shifts among Arctic zooplankton. Marine Ecology Progress Series 493: 291–296.
Kubiszyn, A. M., J. M. Wiktor, J. M. Wiktor Jr., C. Griffiths, S. Kristiansen & T. M. Gabrielsen, 2017. The annual planktonic protist community structure in an ice-free high Arctic fjord (Adventfjorden, West Spitsbergen). Journal of Marine Systems 169: 61–72.
Kuklinski, P., J. Berge, L. McFadden, K. Dmoch, M. Zajaczkowski, H. Nygard, K. Piwosz & A. Tatarek, 2013. Seasonality of occurrence and recruitment of Arctic marine benthic invertebrate larvae in relation to environmental variables. Polar Biology 36: 549–560.
Kumar, S., G. Stecher & K. Tamura, 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874.
Lang, W. H. & A. Ackenhusen-Johns, 1981. Seasonal species composition of barnacle larvae (Cirripedia: Thoracica) in Rhode Island waters, 1977–1978. Journal of Plankton Research 3: 567–575.
Michelsen, H. K., C. Svensen, M. Reigstad, E. M. Nilssen & T. Pedersen, 2017. Seasonal dynamics of meroplankton in a high–latitude fjord. Journal of Marine Systems 168: 17–30.
Mileikovsky, S. A., 1971. Types of larval development in marine bottom invertebrates, their distribution and ecological significance: are–evaluation. Marine Biology 10: 193–213.
Muckenhuber, S., F. Nilsen, A. Korosov & S. Sandven, 2016. Sea ice cover in Isfjorden and Hornsund, Svalbard (2004-2014) from remote sensing data. The Cryosphere 10: 149–158.
Nilsen, F., F. Cottier, R. Skogseth & S. Mattsson, 2008. Fjord–shelf exchanges controlled by ice and brine production: the interannual variation of Atlantic water in Isfjorden, Svalbard. Continental Shelf Research 28: 1838–1853.
Nilsen, F., R. Skogseth & J. Vaardal-Lunde, 2016. A simple shelf circulation model: intrusion of Atlantic Water on the West Spitsbergen Shelf. Journal of Physical Oceanography 46: 1209–1230.
Pradillon, F., A. Schmidt & N. Dubilier, 2007. Species identification of marine invertebrate early stages by whole–larvae in situ hybridization of 18S ribosomal RNA. Marine Ecology Progress Series 333: 103–116.
Pyefinch, K. A., 1948. Methods of identification of the larvae of Balanus balanoides (L.), B. crenatus Brug. and Verruca stroemia O.F. Müller. Journal of the Marine Biological Association of the United Kingdom 27: 451–463.
Rognstad, R. L. & T. J. Hilbish, 2014. Temperature-induced variation in the survival of brooded embryos drives patterns of recruitment and abundance in Semibalanus balanoides. Journal of Experimental Marine Biology and Ecology 461: 357–363.
RStudio Team, 2015. RStudio: integrated development for RStudio, Inc. Boston, MA, http://www.rstudio.com/.
Silberberger, M. J., P. E. Renaud, B. Espinasse & H. Reiss, 2016. Spatial and temporal structure of meroplankton community in a sub–Arctic shelf system. Marine Ecology Progress Series 555: 79–93.
Stone, C. J., 1989. A comparison of algal diets for cirripede nauplii. Journal of Experimental Marine Biology and Ecology 132: 17–40.
Stübner, E. I., J. E. Søreide, M. Reigstad, M. Marquardt & K. Blachowiak-Samolyk, 2016. Year–round meroplankton dynamics in high–Arctic Svalbard. Journal of Plankton Research 38: 522–536.
Thorson, G., 1950. Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews 25: 1–45.
Walczyńska, K. S., M. K. Mańko & A. Weydmann, 2018. Arctic Ocean biodiversity and DNA barcoding – a climate change perspective. In Jungblut, S., V. Liebich & M. Bode (eds), Youmares 8: Oceans Across Boundaries: Learning from Each Other. Springer, New York: 145–153.
Walley, L. J., 1968. Studies on the larval structure and metamorphosis of Balanus balanoides (L.). Philosophical Transactions of the Royal Society of London 256: 237–280.
Ware, C., J. Berge, A. Jelmert, S. M. Olsen, L. Pellissier, M. Wisz, D. Kriticos, G. Semenov, S. Kwaśniewski & I. G. Alsos, 2016. Biological introduction risks from shipping in a warming Arctic. Journal of Applied Ecology 53: 340–349.
Webb, K. E., D. K. A. Barnes, M. S. Clark & D. A. Bowden, 2006. DNA barcoding: a molecular tool to identify Antarctic marine larvae. Deep-Sea Research Part II: Topical Studies in Oceanography 53: 1053–1060.
West, T. L. & J. D. Costlow, 1987. Size regulation in larvae of the crustacean Balanus eburneus (Cirripedia: Thoracica). Marine Biology 96: 47–58.
Weydmann, A., W. Walczowski, J. Carstensen & S. Kwasniewski, 2018. Warming of Subarctic waters accelerates development of a key marine zooplankton Calanus finmarchicus. Global Change Biology 24: 172–183. https://doi.org/10.1111/gcb.13864.
Wickham, H., 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York.
Wiedmann, I., M. Reigstad, M. Marquardt, A. Vader & T. M. Gabrielsen, 2016. Seasonality of vertical flux and sinking particle characteristics in an ice–free high Arctic fjord – different from Subarctic fjords? Journal of Marine Systems 154: 192–205.
Acknowledgements
We would like to acknowledge and thank the following people who assisted in the laboratory, in the field and supported data analysis: Eike Stübner, Miriam Marquardt and Piotr Kukliński. This research was supported by LARVAE – Linking Annual cycles of Reproduction and recruitment to environmental variables in Arctic Epifauna (2014/15/B/NZ8/00237), funded by Polish National Science Centre and MEROPLANKTON - Meroplankton biodiversity, seasonal dynamics and function in high latitude coastal ecosystems, funded by Fram Centre, Flagship Fjord and Coast, Tromsø, Norway.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Dr. Iacopo Bertocci
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Walczyńska, K.S., Søreide, J.E., Weydmann-Zwolicka, A. et al. DNA barcoding of Cirripedia larvae reveals new knowledge on their biology in Arctic coastal ecosystems. Hydrobiologia 837, 149–159 (2019). https://doi.org/10.1007/s10750-019-3967-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3967-y